Introduction
Cardiovascular diseases are the most common cause of death worldwide, particularly in Europe1. In Portugal, cardiovascular diseases accounted for 29.4% of total mortality in 20172. Interventions targeting modifiable risk factors and preventive drug therapies reduce future cardiovascular events. Hence, early diagnosis of cardiovascular diseases has the potential to reduce future morbidity and mortality.
Coronary artery calcifications (CAC) are associated with increased number of major adverse cardiovascular events (MACE), both in symptomatic and asymptomatic patients, and are considered an atherosclerosis biomarker3. Current guidelines recommend CAC scoring as a tool to reclassify cardiovascular risk upwards and downwards in addition to conventional risk factors4.
Cardiovascular (CV) diseases are associated with worse prognosis in COVID-19 patients. Conversely, COVID-19 patients with pre-existing CV diseases are at increased risk of conversion from an asymptomatic subclinical state to unstable disease, with plaque instability and higher risk of acute coronary syndrome5,6,7.
In this study we assess the association between visual CAC scoring on non-gated chest CT examinations of COVID-19 patients and clinical outcomes. In addition, we evaluate the report rate of CAC in this population.
Methods
Population
We prospectively enrolled 105 consecutive RT-PCR COVID-19 positive patients who underwent an ungated chest CT, between April and July 2020. Clinical files were checked for C-reactive protein (CRP) at admission and number of hospitalized days. Clinical endpoints were ventilatory support (invasive and non-invasive) and mortality. Two patients were not hospitalized and were excluded from the clinical endpoint analysis. Taking in account the observational and retrospective nature of this study, the need for informed consent was waived by the ethics committee.
Image analysis
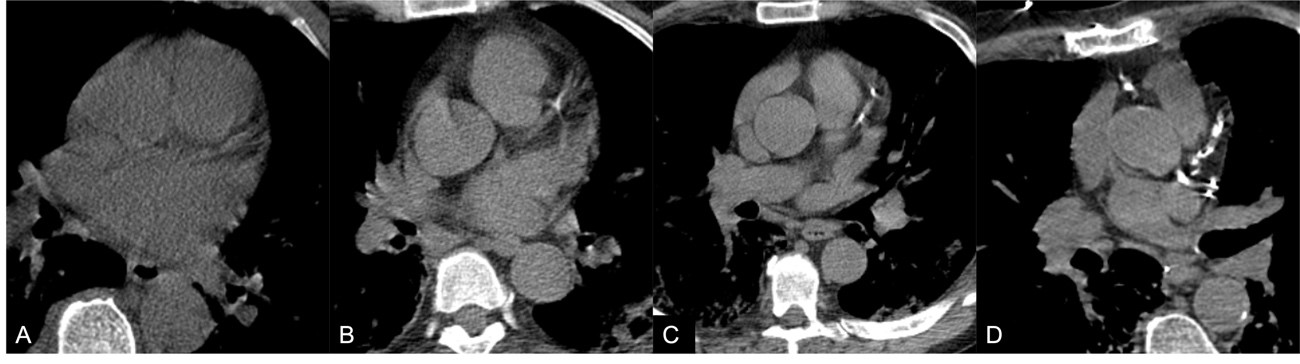
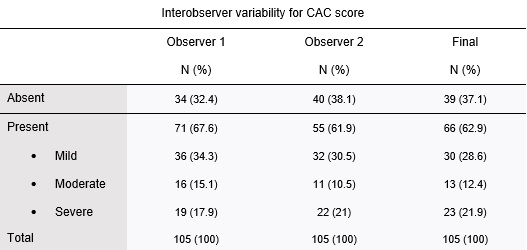
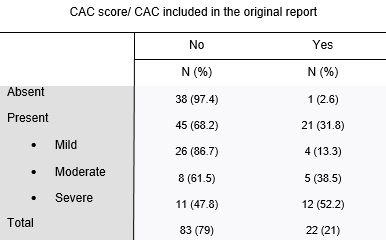
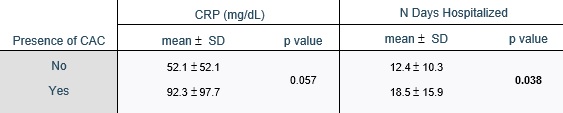
The presence of coronary calcifications was evaluated by two independent observers - observer 1, a radiologist with 8 years of experience in thoracic imaging, and observer 2, with 3 years of experience in thoracic imaging. CAC were scored using a simple visual ordinal score on a whole patient basis, as described in the BSCCT/BSTI guidelines, using the categories absent, mild, moderate, and severe (figure 1 ). Discordant cases were solved by simple consensus. The Kappa coefficient of Cohen was used to calculate the interobserver variability. The original exam reports were reviewed for the mention of CAC.
Statistical analysis
The following statistical tests were used:
T-test: compare the age means in relation to ventilatory support and death;
Chi-square: compare gender differences and presence of CAC across the outcomes;
Mann-Whitney U test: compare CAC scores across the outcomes; difference in CRP values and presence of CAC; difference in hospitalized days and presence of CAC (in patients who survived).
Univariable logistic regression was used to calculate the odds ratio (OR) of needing ventilatory support and death in relation to the presence of CAC.
Statistical significance was defined at 0.05 (two-tailed). Statistical analysis was performed using SPSS 27 (IBM).
Results
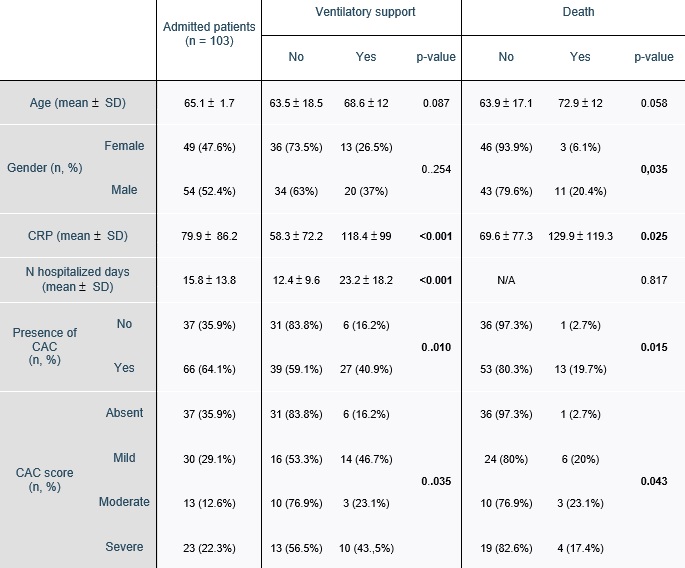
The basic demographic and clinical variables, presence of CAC and CAC scores according to the clinical endpoints (ventilatory support and death) are presented in table 1. The age range was 20-98 years (65.2 ( 16.6, mean ( standard deviation). There was a small male predilection with 54 (52.4%) males and 49 (47.6%) females.
The median hospitalization time was 12 days (IQR: 13 days), ranging from 2 to 82 days. The mean CRP at admission was 77.9 mg/dL (( 86.2 mg/dL).
A total of 33 (32%) of patients needed ventilatory support and 14 (13.6%) died.
Sixty-six patients had CAC on chest CT.
Table 1: Basic clinical variables, presence of CAC and CAC score acording to the clinical endpoints (need for ventilatory support and death). Statistically significant differences are marked in bold.
CAC scoring and reporting
The Kappa coefficient of Cohen for the two observers was 0.72 (standard-error: 0.054). After application of the simple consensus method, 39 (37.1%) patients were classified as having no CAC (absent), 30 (28.6%) as mild, 13 (12.4%) as moderate and 23 (21.9%) as severe (table 2).
The original report rates of CAC according to the different CAC scores are shown in table 3. CAC were reported in 22 (21%) of the original reports and in 21 (31.8%) of the patients classified as having CAC. Report rates were higher for patients with severe calcifications (52.2%) compared to mild calcifications (13.3%) (p<0.001).
CAC and clinical endpoints
Univariable logistic regression results are shown in table 4. Patients with CAC had an OR of 3.6 (p=0.012) of needing ventilatory support and 8.9 (p=0.039) of death.
Table 4: Univariable logistic regression showing the OR for ventilatory support and death in relation to presence of CAC. Significant OR are flagged in bold.

In patients who survived, hospitalization days were higher if they had CAC (p = 0.038). There was not a statistically significant difference in CRP values at admission in relation to CAC (table 5).
Discussion
Imaging indications in COVID-19 patients are controversial and may vary according to country and resource availability. A multinational statement by the Fleischer society recommends chest imaging in patients with COVID-19 and worsening respiratory status and in patients with moderate to severe clinical features of disease and high pre-test probability8. In this way, chest CT is not only an important tool for diagnosis and severity grading, but also to assess extra-pulmonary manifestations7. In fact, our study showed the presence of CAC was associated with worse patient prognosis, particularly by increasing the risk of ventilatory support (OR: 3.6), number of hospitalized days (12.4 days vs 18.5 days) and death (OR: 8.9). Although statistical significance was not achieved, there was also a tendency for higher CRP values in patients with CAC (92.3 mg/dL vs 52.1 mg/dL). Recently published studies have achieved similar results9,10,11 and support the role of CAC as an imaging biomarker for adverse clinical outcomes in COVID-19 patients.
Although the importance of traditional CV risk factors cannot be overemphasized, current evidence shows a significantly increased risk of myocardial infarction in the short, medium and long-term for patients with acute bacterial and viral infections12. In the particular case of COVID-19 infection, three main mechanisms have been described that justify worse CV outcomes7 - multi-organ failure and consequent cardiac injury secondary to severe hypoxia caused by the respiratory syndrome; endothelial dysfunction and myocardium damage due to ACE2 binding; activation of the inflammatory cascade and subsequent cytokine storm. Cardiovascular complications of COVID-19 infection have been extensively described in the literature, and include myocarditis, acute myocardial infarction, heart failure and cardiac arrithmyas6,13,14.,15. Conversely, patients with chronic CV diseases like ischemic heart disease or heart failure are known to have worse prognosis when infected with COVD-1916,17.
The association between CAC and cardiovascular CV disease is well established. During the National Lung Cancer Screening Trial, the hazard ratio (HR) for coronary disease death was 2.09 in the patients with mild, 3.86 with moderate and 6.95 with severe calcifications, when compared to the absence of calcifications18. Also, the visual scoring of CAC was found to be a predictor of all-cause and CV mortality, independent of smoking status19. Visual grading and assessment of CAC is therefore recommended by the SCCT20 and BSTI3 guidelines in all chest CT due to its simplicity, good interobserver variability and correlation with Agatson score. In our study, visual grading of the CAC was reproducible, with a substantial Kappa coefficient (0.72), concordant with the results reported in the literature18.
Thus, visual CAC scoring is a simple and reliable method to provide additional prognostication in COVD-19 patients, available in all patients undergoing chest CT and not requiring additional image post-processing. In addition, it may provide the first indication of coronary artery disease presence.
In our cohort, only 21% of the original reports included information about CAC. However, considering only the patients with CAC, 31.8% were mentioned in the original report, suggesting that denial by omission may have occurred. Previous studies reported similar results - one study in 2013 found that CAC was recorded in the radiologist report of non-contrast chest CT exams in only 44% of the patients with CAC21; another study in 2015 found unreported potentially relevant cardiovascular findings in 39.6% of patients undergoing chest CT for pulmonary interstitial disease, lung cancer staging or PE assessment22.
Our recommendation is to report CAC in all chest CT examinations, whenever they are visible. Specific indications for different CAC grades are currently not defined, and therefore the most important attitude is to report their presence.
Clinical implications of the reported findings were not evaluated, and most physicians may be unaware of the importance of these findings. A recent study found that only 5% of patients with reported CAC on non-gated chest CT had their regular prescription changed23. Nevertheless, the report rate of CAC was low. CT scans of COVID-19 patients were all acquired in the emergency setting. We do not know if in a different setting the result would have been different.
Our study has limitations. First, its retrospective single center nature. Age correction was not performed, and CAC is known to be strongly associated with age. However, age was not statically different in patients who needed ventilatory support, ICU admission or died.
Also, patients with previously known coronary disease were not excluded, which may have introduced an outcome bias as these patients may already be under treatment.
Finally, lung parenchyma involvement by COVID-19 was not considered is this study. A previous study developed an artificial neural network to estimate COVID-19 disease severity, which showed correlation with clinical outcomes24,25. In the future, it would be interesting to incorporate CAC information in this algorithm to further stratify and improve prognostication of COVID-19 patients.