Introduction
Hypertension affects more than 1.3 billion people worldwide.1 Primary aldosteronism (PA) is the most common cause of secondary hypertension,1,2 being present in about 15% of hypertensive patients.3,4 Its prevalence increases with the severity of hypertension.2) The most common causes of PA are aldosterone-producing adenoma or bilateral adrenal hyperplasia.3,4
PA screening is performed by measuring serum aldosterone / renin ratio, with values > 20 being consistent with this diagnosis.1,4,5 A note should be made to very low levels of renin that will lead to false positive results by exaggerating the aldosterone / renin ratio.1 If screening tests are positive, confirmatory tests should be performed by measuring serum or urinary aldosterone levels, after sodium loading.1,4 Persistent elevation confirms autonomous secretion consistent with PA.1,4
Treatment differs depending on unilateral or bilateral autonomous production. When unilateral disease is confirmed, patients can be treated curatively by adrenalectomy. If there is no lateral difference in aldosterone production, medical therapy is used.3,4,5
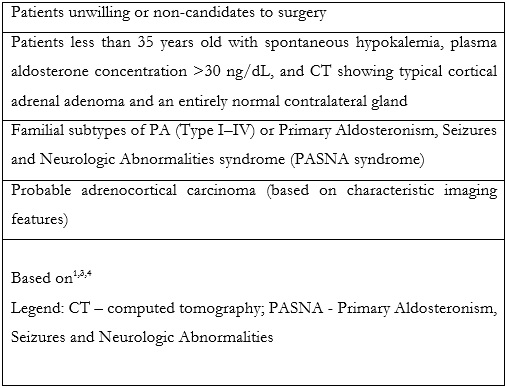
Adrenal vein sampling (AVS) is an established technique, considered the most reliable method for discrimination between unilateral and bilateral adrenal disease.1,3,4,5 Additionally, it can be used for diagnosis of ACTH (adrenocorticotrophic hormone) - independent hypercortisolism (adrenal Cushing syndrome) and for biochemically proven pheochromocytoma, when no apparent cause is visible at computed tomography (CT) or other cross-sectional imaging techniques.3,4,5 Despite that, not all patients are eligible to AVS. Table 1 summarizes the main contraindications for AVS.
According to the Mayo clinic protocol, AVS can be obviated for patients with less than 40 years old, with a unilateral adrenal adenoma with > 1 cm and a normal contralateral gland. These patients can proceed directly to surgery.1,6
Adrenal Vein Anatomy and Potential Pitfalls
The left adrenal vein (LAV) has a caudal or downward path into the superior margin of the left renal vein, where it forms a common trunk of variable length, with the inferior phrenic vein, before the confluence with the left renal vein.4,5 In rare cases (<1%), the LAV drains directly to the left renal vein.4,5
Right adrenal vein (RAV) has 4 to 5 tributaries that converge in a delta configuration to a small (4-5 mm diameter) and short segment that usually has a direct path to the inferior vena cava (IVC), at the level of T11/T12 disk space, in the mid-posterior wall.1,3,4,5 In some patients, it can share a common trunk with an accessory hepatic vein or it can have a direct inferior inflow into the renal vein.1,4 Staining of hepatic parenchymal should be minimal and if prominent, it typically indicates selectivation of small hepatic veins.3 On the right side, presence of multiple adrenal veins has been described and this could be misleading if the vein draining an aldosterone producing adenoma is not sampled.1 On the contrary, some studies have shown that these multiple veins always converge to a central vein that enter the IVC directly.5,7
There is consensus in the literature that RAV cannulation is the most difficult part of AVS,1 with difficulties in achieving consistent technical success and occasional need for re-intervention. The most important finding to keep in mind, when identifying a RAV, is the detection of venous communication with superficial or emissary veins from the adrenal capsule.5 Both glands have superficial, emissary or capsular veins that extend from its surface to the perirenal fat or renal capsule and they are useful markers of a correct position of the catheter.5 On the right side, some of these vessels can communicate with inferior phrenic, intercostal or superficial hepatic veins, as well as with the right renal vein.5 On the left side, they can communicate with the left renal vein, azygos or hemiazygos veins.5
Cross-sectional imaging, ideally CT, prior to AVS allows for evaluation of the vascular anatomy, position of the adrenal glands and size of the IVC.1,4
Adrenal Vein Sampling - Patient Preparation
AVS is considered by Consensus Guidelines of CIRSE a risk group 1 procedure with a low risk of bleeding. Thus, anticoagulant therapy should not be discontinued in general, but the international normalized ratio (INR) value must be less than or equal to 2.0 and the platelet count superior or equal to 50000 per μl.4
Drugs that interfere with the renin-angiotensin-aldosterone system need to be discontinued prior to AVS.1 These drugs include angiotensin converting enzyme inhibitors, angiotensin receptor blockers and mineralocorticoid receptor antagonists.1 Timing for suspension varies among literature, with some authors indicating a two-week suspension prior to AVS and others eight weeks.1,8,9 Antihypertensive drugs that can be used alternatively include doxazosin, hydralazine, diltiazem and nifedipine.1 Also, patients should follow an unrestricted sodium diet, since a low sodium diet leads to bilateral aldosterone secretion and may affect laboratory measurements.1 AVS must be performed in the morning, due to the circadian fluctuations in aldosterone levels, and during fasting, to avoid changes in serum aldosterone concentrations caused by food intake.4
Adrenal Vein Sampling - Technique
AVS is usually performed in a sequential manner, where the right adrenal vein cannulation is done first, since it is more time consuming and by doing so time gap between samplings can be reduced.1 Another alternative is to perform AVS of the right and left adrenal veins simultaneously, with bilateral femoral accesses. This approach is more time-consuming and has not been established as superior to the sequential technique.4 Also, super-selectivity of different adrenal sub-segments to detect hyperfunctional areas is clinically not relevant and has a poor cost-benefit ratio.4 Adrenal vein cannulation is usually performed after collecting blood samples from the inferior vena cava for hormonal sampling. Usually, 5-6 mL of blood is enough for the inferior vena cava and for each adrenal vein. Another option is to obtain a peripheral vein sample for each adrenal vein sample collected.
Some centers adopt perfusion of a synthetic peptide of the adrenocorticotropic hormone (ACTH) to stimulate adrenal glands, 30 minutes prior and during AVS procedure.1,4 ACTH-analogous peptide induces a 5 to 10 time increase in adrenal vein cortisol levels, while peripheral cortisol levels remain relatively stable. By doing so, this increases a ratio known as the selectivity index (SI) from 2 to 5.1 A potential misleading effect of ACTH-analogous administration is that it can cause stimulation of the normal adrenal gland and have a variable, sometimes minimal, effect on the aldosterone producing adenoma, causing a falsely non-lateralizing study.1 As such, the literature is still not clear about the benefits of synthetic peptide stimulation in the diagnostic accuracy of AVS, and so it is not routinely used.4
There are no guidelines regarding the type and size of catheters recommended, where Cobra, Simmons and Vertebralis catheters are the mostly used ones.1,4 It is generally difficult to retrieve sufficient venous sample in the RAV, given its small diameter prone to temporary collapse and occlusion, preventing backflow of blood when aspirating. The presence of a side hole, placed 3 mm from the tip of the catheter, helps to obviate this problem and to decrease risk of thrombosis.1,5 Other possible techniques to minimize this risk include intermittent and gentle suction; suction with air inside the syringe to reduce the effective suction pressure, or passive/free drainage.5
Care should be taken when using contrast medium as it can interfere with the measurement of serum aldosterone levels, and it can increase the risk of adrenal vein hemorrhage/damage by increasing pressure during injection.1,4 In this manner, the first 2 to 5 cc of aspirated blood should be wasted.1 Some authors advocate free contrast AVS to minimize serum aldosterone level changes.4
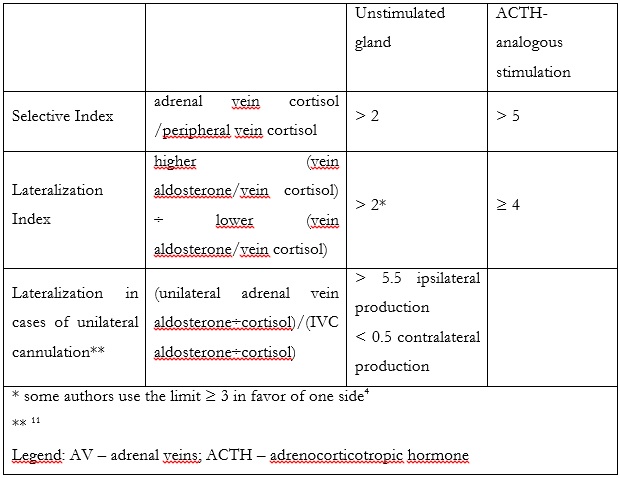
To assess correct AVS, 2 indexes should be measured. The first one is called SI and indicates correct adrenal cannulation by demonstrating a gradient between the peripheral and adrenal vein cortisol levels. Indices superior to 2 and 5 are used as cutoff values in unstimulated and stimulated sampling, respectively.1 To assess lateralization in the production of aldosterone, Lateralization Index (LI) is calculated (table 2). A ratio of > 2 for unstimulated and > 4 for stimulated sampling is recommended by the Endocrine Society.1,10
In cases when only one vein is successfully cannulated, Pasternak et al. described a formula [(unilateral adrenal vein aldosterone÷cortisol)/(IVC aldosterone÷cortisol)] that may predict lateralization. A value of > 5.5 accurately predicted ipsilateral production, while values < 0.5 predicted contralateral production (100% specificity and 50% sensitivity). Values in-between contained cases of both bilateral and unilateral secretion.1,11
Other possible indices include the contralateral index (nondominant adrenal vein vs inferior vena cava A/C)(12)and aldosterone index (aldosterone level in adrenal vein / aldosterone level in the inferior vena cava) used to assess selectivity - if < 1.5, it predicts contralateral suppression.2,13,14
With time and practice, AVS can become a safe procedure, with low complication rates and acceptable technical success. Rossi et al. reported, in their largest published multicenter observational study, a major complication rate between 0.51% and 0.61% (16/2604).1,15 The major complication described is rupture of the adrenal vein, with possible subsequent intraglandular and widespread periadrenal hemorrhage.5 Other possible, but rare, complications include adrenal vein thrombosis, gland or perigland hematoma, or adrenal infarction.1,4,5
Due to the technical difficulties of execution, especially RAV cannulation, AVS has low usage among hospital centers.2,4 The learning curve is estimated to be around 20 to 30 cases, with a maintenance of about 15 annual cases to achieve satisfactory results.1,16 In low volume centers, it is advised that AVS should be performed by a single dedicated interventional radiologist.1
Adrenal Vein Sampling - How we do it
Patients are admitted to the ward the day before procedure. Evaluation of regular medication and allergies is carried out; laboratory work is requested to analyze platelet count, kidney function and coagulation status. A pre-procedural fasting of 4h is required. Perfusion of intravenous tetracosactide is started 30 minutes prior to procedure (50micro/h).
A variety of catheters may be used. The authors prefer a femoral access and the 4 Fr Berenstein for left adrenal vein and either 5Fr Cobra, Simmons or Michaelson for right adrenal vein sampling (Figure 1a). Catheter preparation starts with flushing and then piercing the tip at about 3 mm proximal to the end orifice to create a side-hole (Figure 1b and 1c). More than one hole can be made, but it may render the catheter unusable. The hole must be big enough to prevent vein collapse and allow for adequate flow when aspirating the blood for sampling; we prefer to use a dilution needle. First, we harvest 5 ml of venous blood from the infra-renal IVC with the Berenstein catheter that is going to be used for left AVS. The sample is divided in 2 tubes. A hydrophilic 0.035´´guidewire is used for left renal vein catheterization. The tip of the Berenstein catheter is turned upwards and the catheter is pushed until it locks in the common adrenal-phrenic vein trunk (Figure 2). Cautious injection of contrast shows the confluence of both veins and confirms that the catheter is in place. Aspiration of 5 ml of venous blood is performed and placed in 2 tubes that are sent to the laboratory for immediate evaluation. Selective catheterization of the LAD past the phrenic branch is attempted but it is not mandatory.
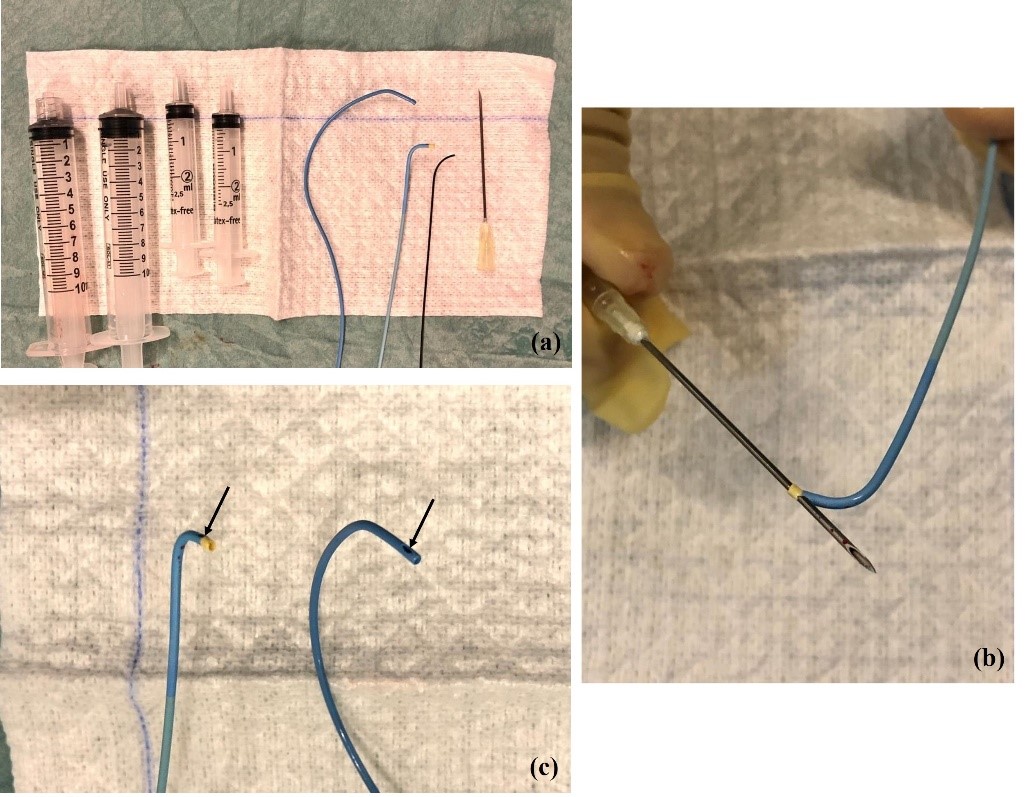
(a) From left to right: 10 mL syringe with and without Luer lock; 2,5 mL syringes; Cobra 4 Fr catheter, Berenstein 4 Fr catheter, Hydrophilic guidewire; dilution needle (22 gg). (b) Piercing of Berenstein catheter with dilution needle to make a lateral hole and (c) end-result (Berenstein catheter on the left side and Cobra 1 catheter on the right side).
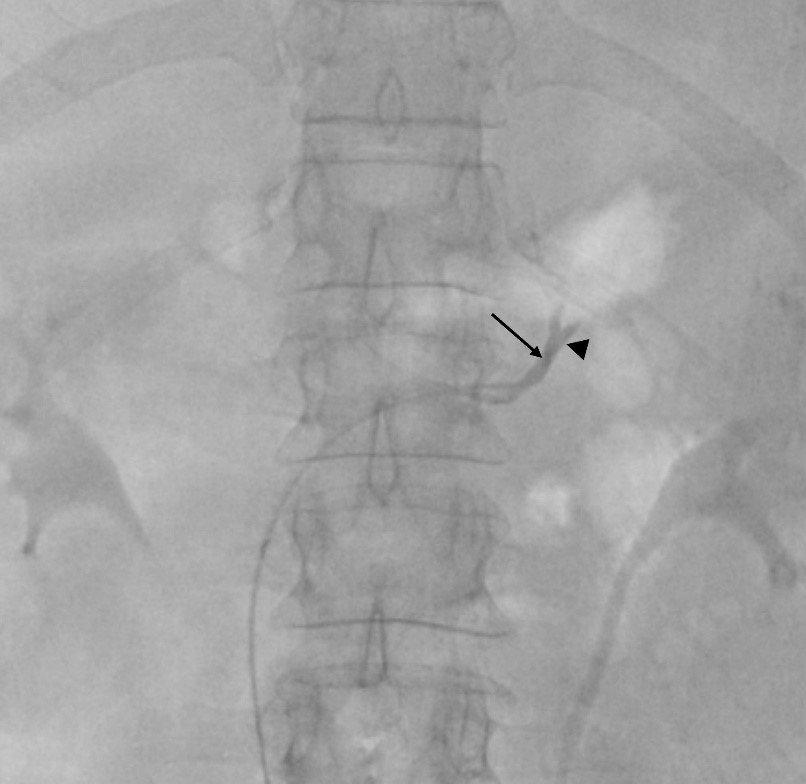
Figure 2: Common adrenal-phrenic vein trunk fluoroscopic presentation. The tip of the Berenstein catheter (arrow) is turned upwards and locked in the common adrenal-phrenic vein trunk (arrowhead).
Detection of the right adrenal vein is usually more challenging (Figure 3); several variants have been described in the literature and there are many pitfalls that the interventional radiologist needs to consider, as stated before (Figures 4, 5 and 6). Oblique fluoroscopic views may help to determine if the catheter is facing forward or backward and rule out pitfalls, such as catheterization of the caudate lobe vein.
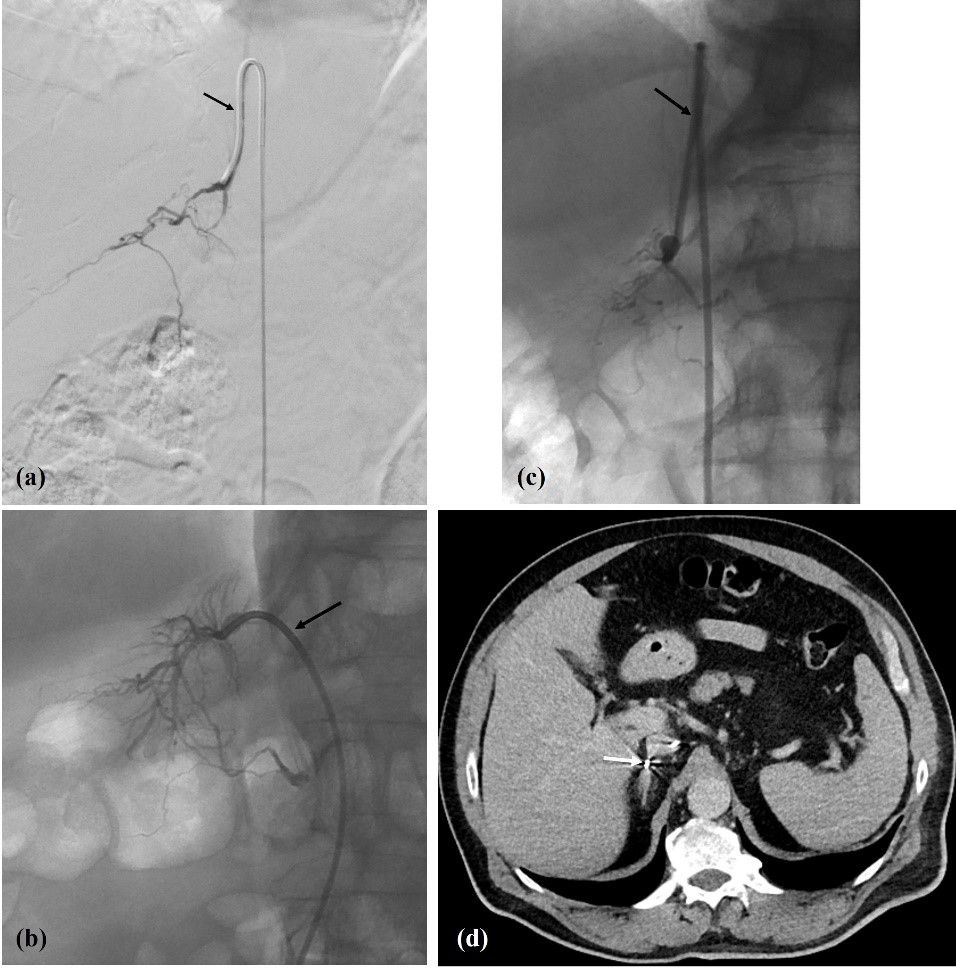
Figure 3: (a, b, c) Right adrenal vein fluoroscopic presentation (arrows: a - Simmons 1 catheter; b - Cobra 1 catheter; c - Simmons 1 catheter); and (d) intra-procedural CT (axial slice) for confirmation (arrow - catheter tip inside RAV). In (b) note the typical delta shape configuration of RAV.
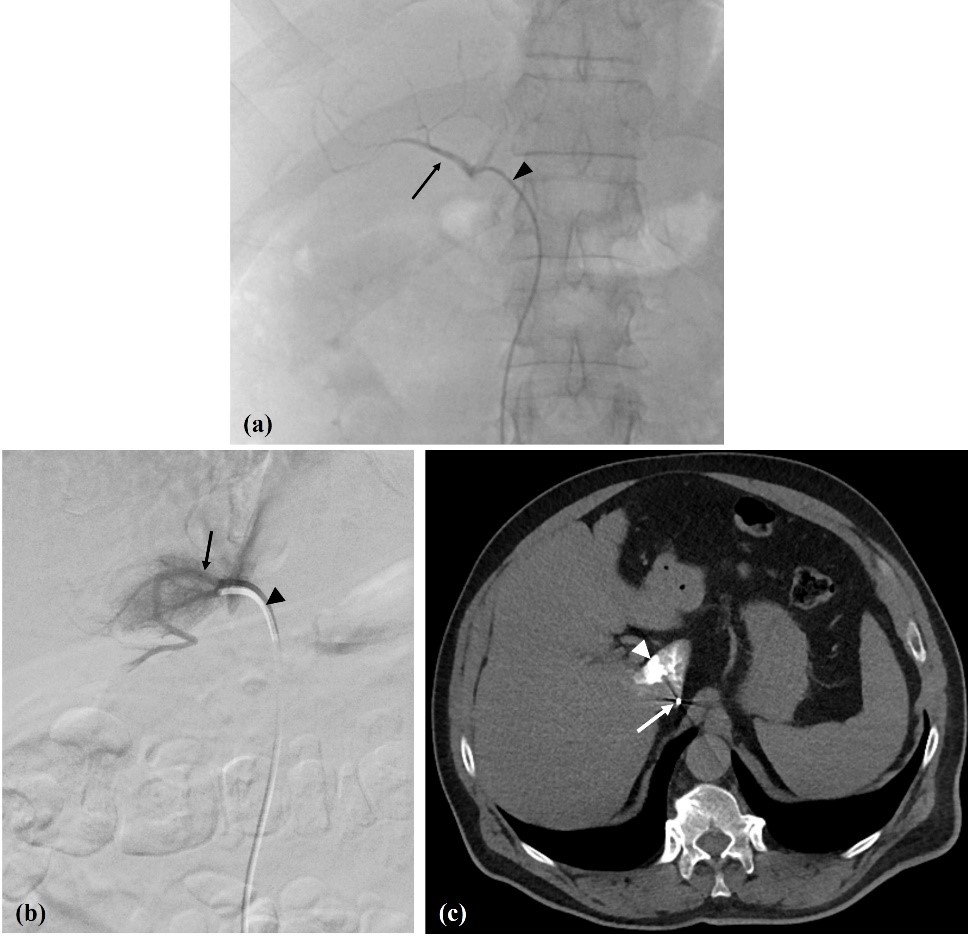
Figure 4: Pitfalls. (a) Inferior accessory hepatic vein (arrow); Cobra 1 catheter - arrowhead. (b) Caudate lobe vein (arrow); Berenstein catheter - arrowhead, and (c) catheter position confirmed with venous CT (arrow) with some caudate lobe staining (arrowhead).
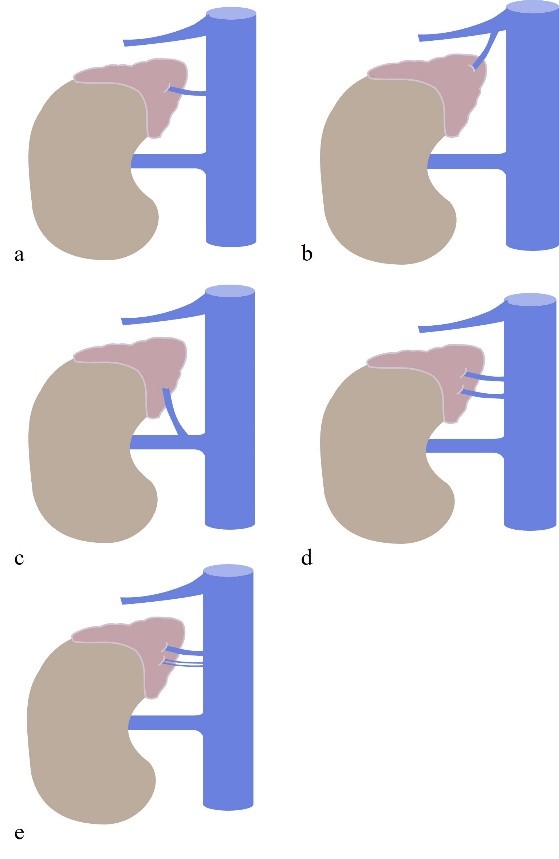
Figure 5: Right Adrenal Vein anatomy and variants. (a) normal anatomy. (b) RAV drains to the right hepatic vein; (c) RAV drains to the right renal veins; (d) double RAV draining directly to the ICV; (e) triple RAV, all draining directly to the ICV. IVC = inferior cava vein.
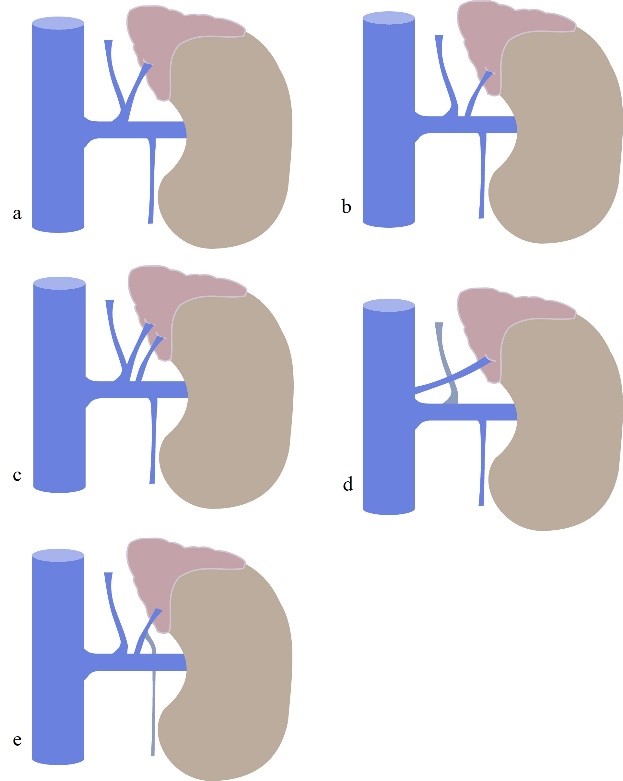
Figure 6: Left Adrenal Vein anatomy and variants. (a) normal anatomy. (b) LAV drains to the left renal vein; (c) double LAV one receiving inferior phrenic vein; (d) LAV draining directly to the ICV; (e) LAV receiving left gonadal vein. ICV = inferior cava vein.
Cannulation of a vein at or near the supposed level (T11-T12) and demonstrating typical fluoroscopic features with soft injection of contrast warrants immediate harvest of venous blood and placement in 2 tubes. If the vein collapses or there is otherwise no flow, slight adjustments in position can be made, with subsequent confirmation that cannulation is still in place. It is also important to make sure that the side-hole created in the catheter is enough to allow gentle aspiration of blood during wedged venous catheterizations. We recommend that other veins incidentally cannulated at or near the expected level of the RAV be sampled as well, even if they do not show the typical fluoroscopic features.
Previous contrast-enhanced CT may help with venous mapping, but it does not affect technical success. The authors have performed intra-procedural CT to check for catheter positioning in some cases (Figure 3d), but it doesn’t increase overall technical success and may lead to false positives.
Currently we are performing all AVS procedures in close coordination with the laboratory and endocrinology departments. We only remove the femoral access sheath after laboratory confirmation of correct bilateral catheterization through cortisol measurement. Unilateral catheterization is considered insufficient by many; thus, it is essential to certify a correct bilateral catheterization. Due to the absence of pathognomonic imaging findings of the RAV, we prefer to wait for the laboratory results to confirm the technical success, while the patient is still in the Interventional Radiology unit. With the patient in the angiography suite, we collect the blood samples from the inferior vena cava and both adrenal veins and send the products to the laboratory department. As it may take between 30 to 60 minutes to have a laboratory confirmation of bilateral technical success, we then remove the catheters and only leave the femoral access sheath in place while the patient is taken into the recovery area and waits for the laboratory confirmation. If the laboratory results show a technical success the femoral sheath is removed, and manual femoral compression is performed for 10 minutes. If the laboratory results show a technical failure (usually due to failed RAV catheterization), the patient is sent back to the angiography suite and RAV sampling is attempted again. This approach has allowed us to improve our technical success rate which was 40% when fluoroscopic and CT guidance were used and is now 100% with laboratory confirmation.
Conclusion
AVS is a safe and feasible procedure, with low risk of complications. Due to the technical difficulties of execution, especially RAV cannulation, AVS has low usage among hospital centers. The learning curve is estimated to be around 20 to 30 cases, with a maintenance of about 15 annual cases to achieve satisfactory results. In low volume centers, it is advisable that AVS should be performed by a single dedicated interventional radiologist. Right AVS remains a challenge, and the key to success comes with the good choice of materials, experience, and close communication with the laboratory department during the procedure. Our technique, which is adapted from the current literature, has improved from a 40% technical success rate when only imaging was used for confirmation to 100% after laboratory confirmation.