Introduction
Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC) are the most common primary liver malignancies. However, the synchronous occurrence of both in the same liver is very rare.1
A long way back Allen and Lisa recognized 3 categories of concurrent HCC-CC, namely those appearing as separate nodules, contiguous but independent, masses or an intermingling of hepatocellular and glandular elements.2 The latter two are considered a form of combined HCC-CC, whereas the former is separately regarded as synchronous double primary HCC and intrahepatic CC. Both tumors are associated with poor prognosis and the early diagnosis and management is crucial in prognosis.3 Given the rarity of their synchronous incidence in the same liver as separate nodules, currently available literature is scarce.1
We report a rare case of synchronous development of HCC and CC in a patient with alcohol-induced liver cirrhosis.
Case Presentation
A 59-year-old man was referred from another institution to a Liver Transplantation (LT) consultation, diagnosed with decompensated alcohol-induced liver cirrhosis. He had ascites controlled with diuretics, persistent grade 1 type C hepatic encephalopathy and portal hypertension complicated with esophageal varices. Alcoholic etiology was inferred given the heavy habits of alcohol consumption over several years and the exclusion of other etiologies.
His Model for End-stage Liver Disease (MELD) and Child-Pugh Scores were 11 and 8, respectively. Other relevant personal medical conditions included type-2 diabetes mellitus and atrial fibrillation.
Physical and mental examination revealed significant muscular atrophy and depressed humor Laboratory investigation revealed low platelet counts, hypoprothrombinemia, and elevation of total bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase levels. Serum alpha-fetoprotein (AFP) was normal.
Abdominal ultrasound (US) revealed an isoechoic nodule measuring 2.8 cm on segment III of the liver. Subsequent Computed Tomography (CT) showed two hypervascular nodules, namely the one described on US and another on segment IVa, measuring 1.3 cm. Both showed nonperipheral wash-out on the late phase of enhancement (Fig. 1), therefore being classified as LR-5 (definite HCC), according to Liver Imaging-Reporting and Data System.
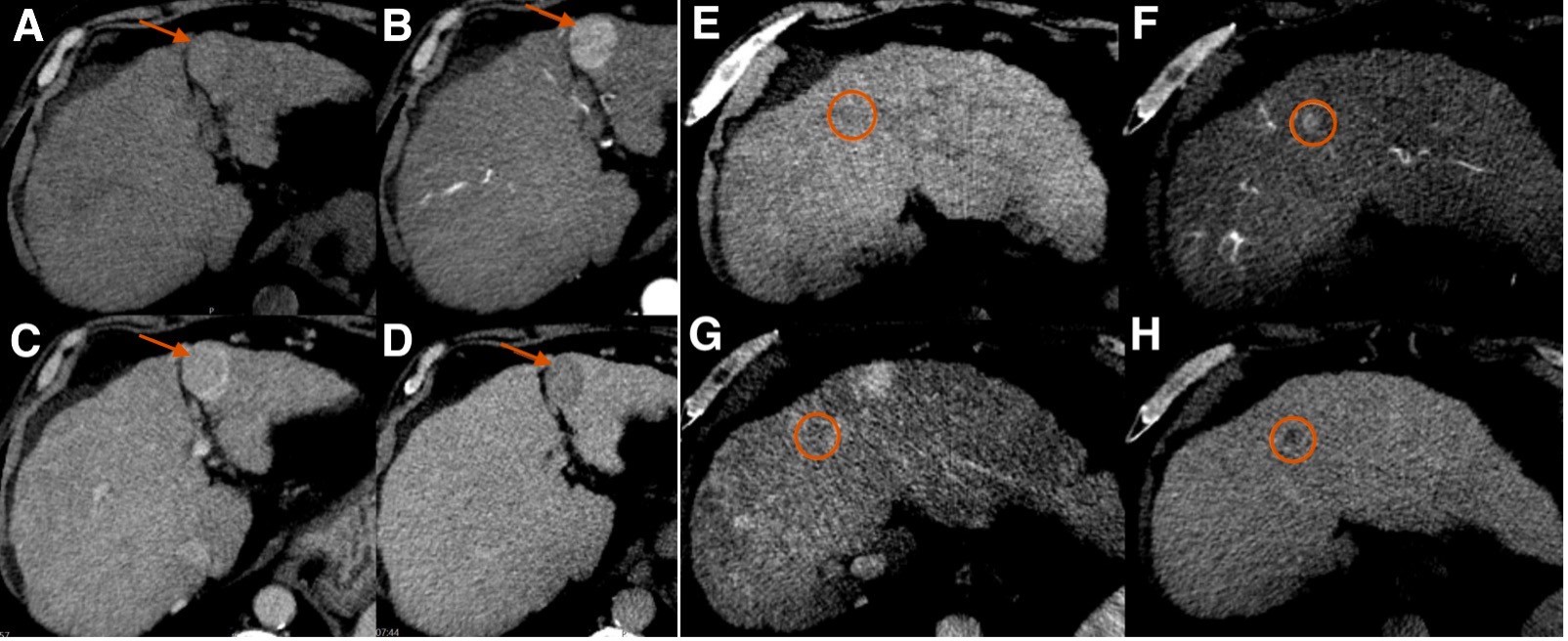
Figure 1: Unenhanced (A, E) and contrast-enhanced axial abdominal Computed Tomography images in the arterial (B, F), portal venous (C, G), and delayed venous (D, H) phases of opacification, showing a 2.8 cm nodule (indicated by the arrow) on segment III (A-D) and a 1.3 cm nodule (circled) on segment IVa (E-H), both with non-rim arterial phase hyperenhancement and nonperipheral washout in the delayed phase.
Following the Barcelona clinic liver cancer staging system algorithm for management of HCC, our patient met the criteria for LT, as he only had 2 nodules measuring less than 3 cm, his performance status was 0 and he had no contraindications to LT. Meanwhile, transarterial chemoembolization (TACE) with doxorubicin of the larger nodule was performed, to prevent it from getting larger and no longer meet LT criteria.
In the subsequent month, the patient was placed on the national LT waiting list and was kept under clinical and imaging surveillance. Follow-up CT performed 1 month after TACE showed no signs of viable tumor on segment III, and the smaller nodule was stable. Six months later the segment III was still tumor-free, but the smaller nodule showed slight enlargement and was also submitted to TACE, as were two other smaller hypervascular nodules detected on angiography. Follow-up CT 1 month later showed no enhancement in the embolized area of segment IVa, nor in the segment III, consistent with complete response.
Follow-up CT 4 months later was unremarkable. However, Magnetic Resonance Imaging (MRI) 7 months after TACE showed a focus of arterial hyperenhancement with subsequent late wash-out within the nodule located in the segment III, suggestive of viable tumor (Fig. 2A-D). The nodule in the segment IVa measured less than 10 mm, being hard to characterize. It was heterogeneously hyperintense in the arterial and portal phases of opacification with subsequent washout-out in the transition phase, remaining hypointense in the hepatobiliary phase (Fig. 2E-H).
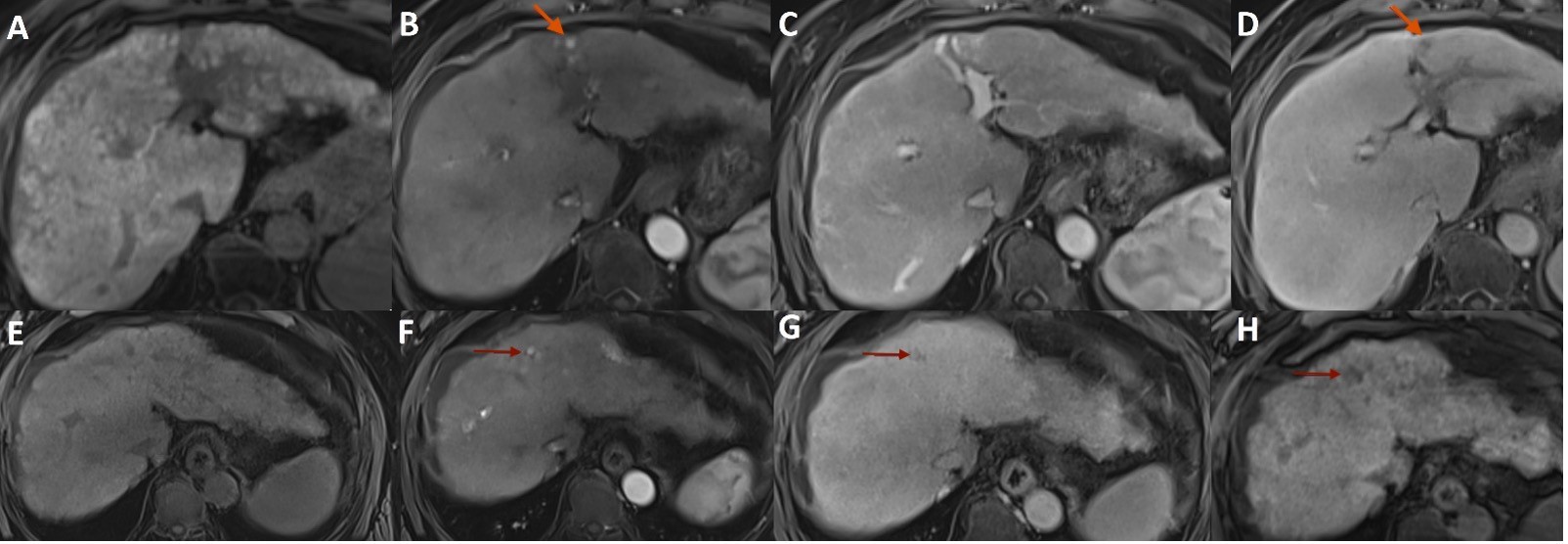
Figure 2: Abdominal MR axial T1-weighted images with fat saturation before (A,E) and after administration of hepatobiliary contrast agent in the arterial (B,F), portal (C,G), and transition (D,H) dynamic phases showing a focus of arterial hyperenhancement (arrow in B) with subsequent late wash-out (arrow in D) within the nodule located in the segment III, suggestive of viable tumor, and a small nodule in the segment IVa (arrow in F-H), heterogeneously hyperintense in the arterial phase of opacification with subsequent washout-out in the transition phase and remaining hypointense in the hepatobiliary phase.
At multidisciplinary team meeting, it was decided to schedule a new TACE of the nodule with recurrent viable tumor in segment III. Fortunately, within less than two months the patient received an LT.
At microscopic analysis, the explant liver was cirrhotic. In segment III a well-demarcated nodule with a fibrous capsule was found. Microscopically, this segment showed coagulative necrosis foci around chemoembolization spheres with neighboring foreign body reaction, fibrosis, and hemosiderin. In the adjacent areas, foci of viable residual neoplasm with a trabecular and pseudoglandular pattern were found, consistent with HCC, without lymphovascular invasion (Fig. 3A). Immunohistochemical staining was negative for cytokeratin (CK) 19. Segment IV harbored a fibrosed subcapsular area, in which a multilobulated and irregular neoplasm with glandular elements and hyaline stroma was found, without vascular invasion. These features were consistent with CC, further substantiated by strong and diffuse positive CK7 and CK19 staining, and negativity for HEP Par (Fig. 3B-E). Both nodules were moderately differentiated (G2). These nodules were in the same location as the ones detected on imaging and submitted to TACE, and their size was concordant. In addition, regenerative nodules were found in the specimen.
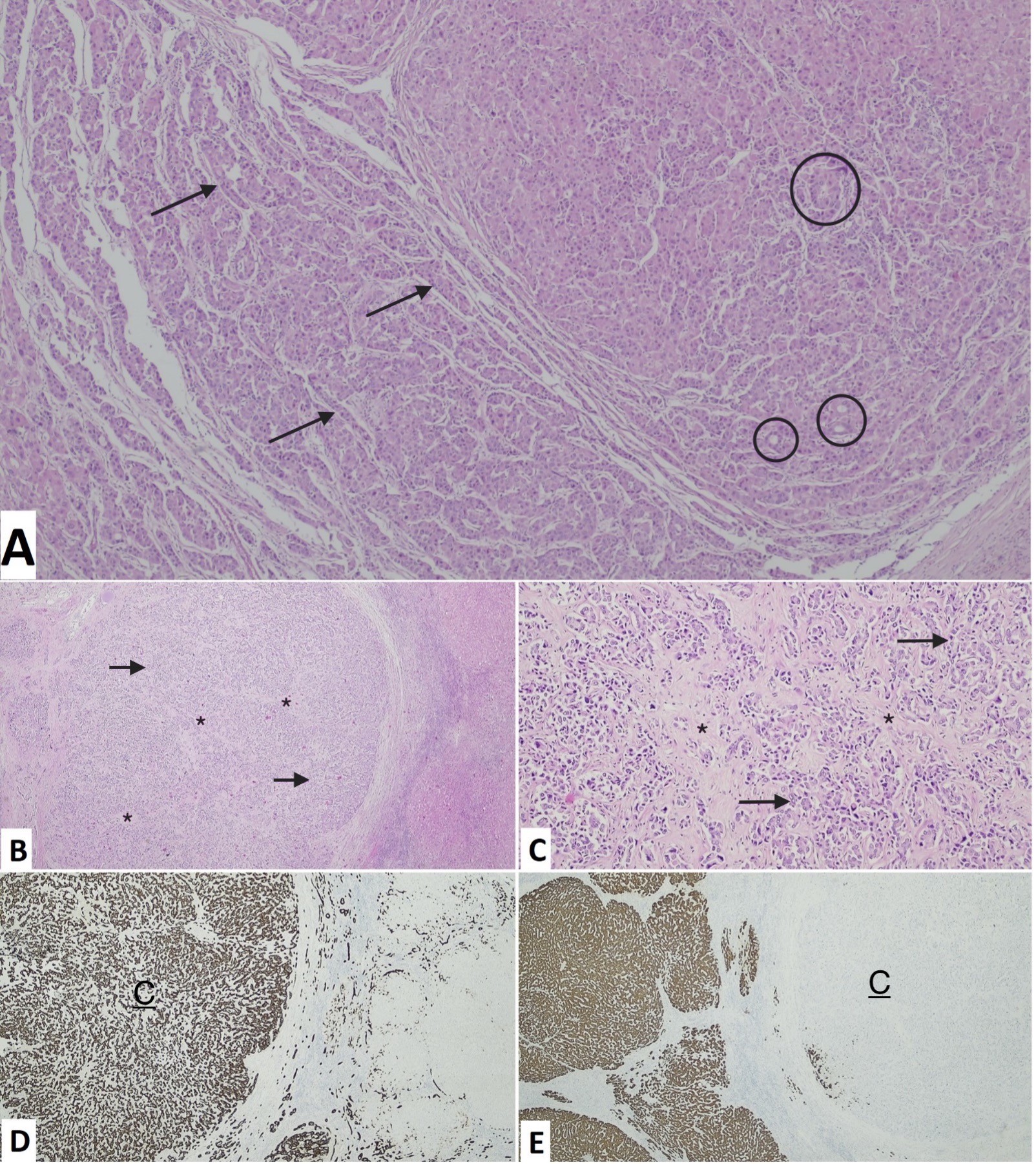
Figure 3: Light-microscopic features of hepatocellular carcinoma in segment III (A, hematoxylin and eosin staining, 40x) with trabecular (arrows) and pseudoglandular (circles) growth patterns, and cholangiocarcinoma in segment IV (B, hematoxylin and eosin staining, 40x; C, hematoxylin and eosin staining, 100x), with glandular elements (arrows) and hyaline stroma (*). Immunohistochemical analysis of cholangiocarcinoma is positive for CK19 (D, 40x) and negative for HEP Par (E, 20x).
During follow-up, the patient was stable and reported no symptoms. Histological and imaging evaluations of the transplant liver were unremarkable. Laboratory studies, including serum tumor markers AFP and carbohydrate antigen 19-9 (CA19-9), remained normal.
Discussion
HCC is by far the most common primary liver malignancy, accounting for more than 80%. CC is the second most common. Despite that, the synchronous development of both these entities is extremely rare.4 It has been suggested that progenitor cells can differentiate into hepatocytes as well as cholangiocytes.5
Given the scarcity of synchronous double primary HCC-CC, its diagnosis is very difficult preoperatively before the pathological confirmation. Both nodules detected in our patient were considered suggestive of multiple HCCs on imaging studies prior to definitive pathological diagnosis in the explanted liver, as was the case in previous reports.1,6
HCC commonly develops in patients with chronic liver diseases of multiple etiologies. Risk factors for CC include cirrhosis, primary sclerotic cholangitis, parasitic infections, biliary duct cysts, and others.6,7 Several studies have demonstrated an important role of chronic liver inflammation in the coincidental development of double HCC-CC.1,8
As clinical features of both HCC and CC are unspecific and sometimes occult, serum tumor markers (namely AFP for HCC and CA19.9 for CC) and imaging studies are key tools to establish the diagnosis.1 Synchronous HCC and CC may have an elevation of both AFP and CA19-9, although they are neither sensitive nor specific enough to diagnose them.1,3 The case of our report had persistently normal AFP levels, as is the case of almost one-third of HCCs.10 Carr et al found AFP levels <20 IU/ml in 41.9% of HCCs, most smaller and much less aggressive than those with elevated levels.11 CA19-9 levels were not measured initially in our case, as CC wasn’t considered.
B-mode ultrasound shows low specificity and accuracy for characterization of small liver lesions, whose characterization is even more difficult on a cirrhotic background.12 Liver lesions detected on US should be further characterized with contrast-enhanced US, CT, or MRI.7
The hallmark features of HCC on CT are those of a nodule showing arterial phase enhancement followed by a washout in the portal and/or delayed phase, surrounded by a pseudocapsule. Internal mosaic pattern, intralesional fat, and vascular invasion (especially portal vein) are also typical. On MRI, most HCCs are mildly hyperintense or isointense to the liver on T2-weighted images, and hypointense on T1-weighted images. Although MRI is better than CT for evaluating the heterogeneous appearance of large HCCs, the diagnosis is essentially based on dynamic post-gadolinium-enhanced images. Enhancing pattern is the same as described for dynamic CT. Diffusion-weighted imaging is very sensitive for the detection of nodules, although ADC values are not specific for differentiating HCC from dysplastic nodules or other malignant and benign lesions. Still, when combined with dynamic MRI, it increased the sensitivity in the appreciation of small HCCs.13
Histologically, HCCs are typically well-vascularized tumors with wide trabeculae, cytologic atypia, mitotic activity, vascular invasion, and absence of Kupffer cells and reticulin network. They are mostly encapsulated and lack portal tracts. Its most common histologic growth patterns are trabecular, pseudoglandular, and solid. Regarding immunohistochemistry, CK7 and CK19 stains are mostly absent in HCC. CD34 staining is diffuse and sinusoidal, as opposed to peripheral and focal in high-grade dysplastic nodules. Hep Par 1 antibody is a useful marker for hepatocellular differentiation.14
Mass-forming CC typically appears as a lobulated non-encapsulated homogeneous sclerotic mass. Histologically it is composed of central fibrosis and coagulative necrosis with scanty scattered tumor cells intermingled, surrounded by a rim of tumoral cells. Portal venules, lymphatics, and nerves are often precociously invaded.14,15 Tumor cells are positive for CK7 and CK19, although no specific markers are yet available.15
When indicated, surgical resection is the treatment of choice for synchronous HCC-CC.1 LT is an alternative approach in cirrhotic patients who meet the inclusion criteria. Loco-regional therapies for HCCs have been used as bridging therapy to maintain the tumor burden within criteria. The most used include TACE (predominantly), radioembolization, thermal ablative therapy, and stereotactic body radiation therapy, none of them recommended over another.16) Neoadjuvant therapies can also be used to reduce the tumor burden of locally advanced intrahepatic CC to allow eventual resection or LT, although further clinical trials are warranted to recommend them.17
The prognosis of synchronous HCC-CC is often recognized as being worse than that of either HCC or intra-hepatic CC alone, given its ability to recur and metastasize early. In a retrospective analysis of 35 of synchronous HCC-CC, the recurrence rate found was 77.1% (median time of 8 months) and 20% had distant metastases after partial hepatectomy. Only CC originated lymph node metastasis. The median overall survival was reported as 18 months, independently affected by the tumor size, histological differentiation of the CC component, and presence of lymph node metastasis.1,3 Other retrospective study found recurrence in 38 of 50 cases (76%), mostly intrahepatic and highest between 3 and 6 months, gradually declining after 9th months. Median disease-free survival (DFS) and overall survival times were 10 and 21 months, respectively. Interestingly, only the size of CC affected DFS, namely through lung metastasis, consistent with its biological behavior. Such findings support a greater influence of CC in worsening the prognosis and survival in these patients, reinforcing the need of prompt diagnosis and a more aggressive management of the CC in comparison with HCC.9)
In conclusion, this article illustrates an exceptionally rare case of simultaneous HCC and CC in a cirrhotic patient. The CC was unsuspected until liver resection for transplantation. Although rare, it is important to be aware of the potential occurrence of these synchronous entities in cirrhotic livers, for which timely directed management is crucial to improve survival. It also reinforces the potential great value of locoregional therapies in eligible patients with HCCs that wait a long time for LT and otherwise would evolve, hindering this potentially curative treatment and limiting survival.















