Case description
A 63-year-old-female patient presented with multiple palpable nodules in both breasts. She had a clinical history of systemic sarcoidosis. Physical examination revealed multiple palpable indurations in both breasts. There were no skin changes nor nipple discharge. No axillary or supraclavicular lymphadenopathy were noted.
Bilateral mammography (Fig. 1) showed multiple oval high-density masses, with microlobulated margins, measuring less than 15 mm, in all quadrants of both breasts (BI-RADS 4b), without calcifications. Ultrasonography (Fig. 2) showed multiple oval, hypoechoic, heterogeneous solid masses with microlobulated margins, parallel to the skin (BI-RADS 4b). A left axillary lymphadenopathy was noted at ultrasound examination.
MR (Magnetic Resonance) imaging was performed to determine the extension of the disease and to identify target lesions for an accurate biopsy. MR imaging revealed several oval hypointense masses on T1 and T2-weighted images with circumscribed margins in both breasts. These masses demonstrated homogeneous enhancement on dynamic contrast-enhanced fat-saturation T1-weighted MR imaging. Kinetic curves study (Fig. 3) showed some masses with rapid initial uptake followed by a plateau phase (Type 2 curve) and others showed a rapid enhancement with washout pattern (Type 3 curve), both being considered of concern for malignancy.
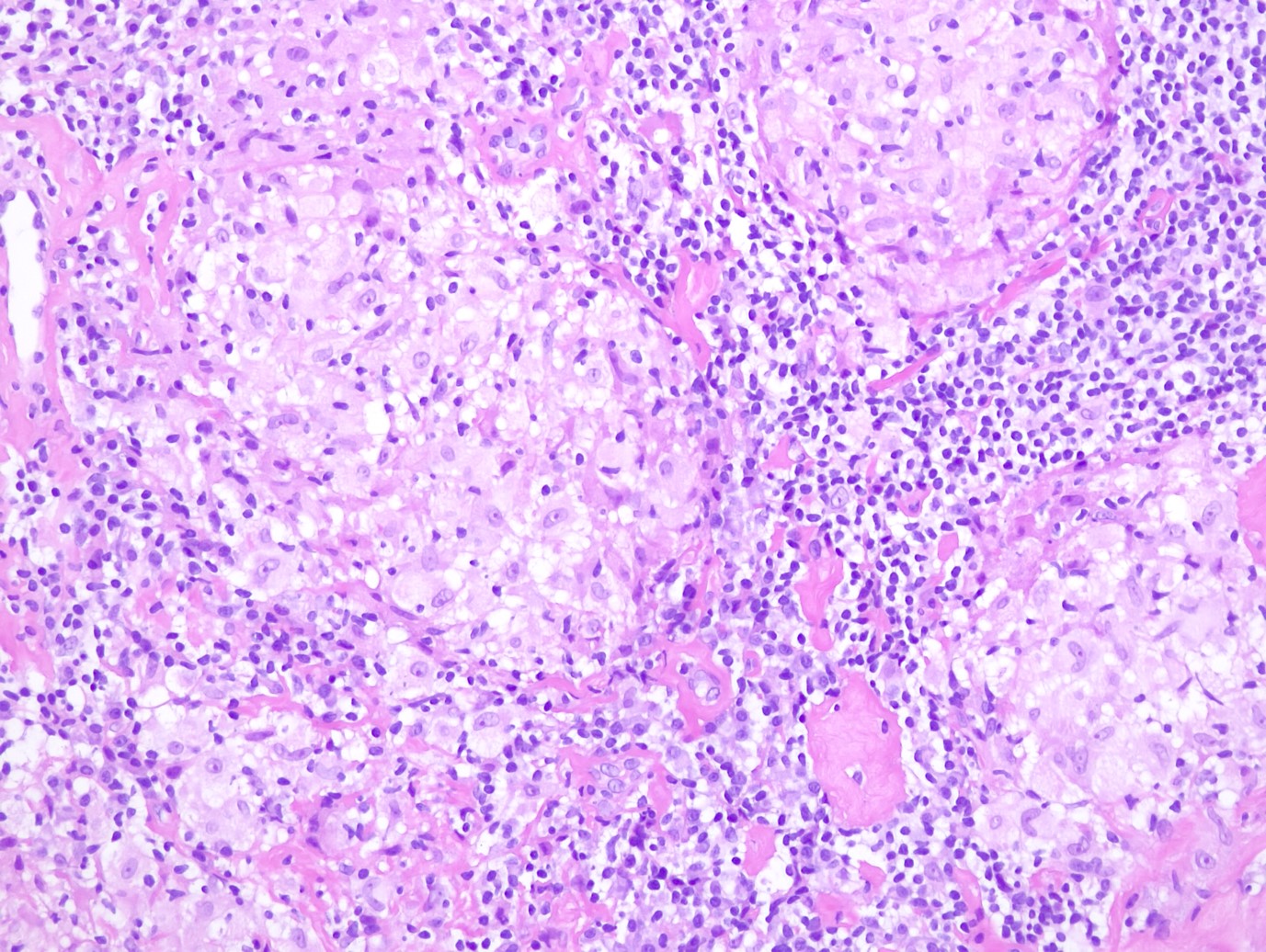
Physical examination and imaging results were inconclusive to establish a definitive diagnosis and they were unable to exclude malignancy. Therefore, the patient underwent 14-Gauge core-needle ultrasound guided biopsies on both breasts, targeting the most suspicious lesions. Histological examination revealed multiple non-caseating granulomas (Fig. 4). Ziehl-Neelsen test was negative for the presence of mycobacteria, ruling out tuberculosis. The final histologic diagnosis was sarcoidosis of the breast.
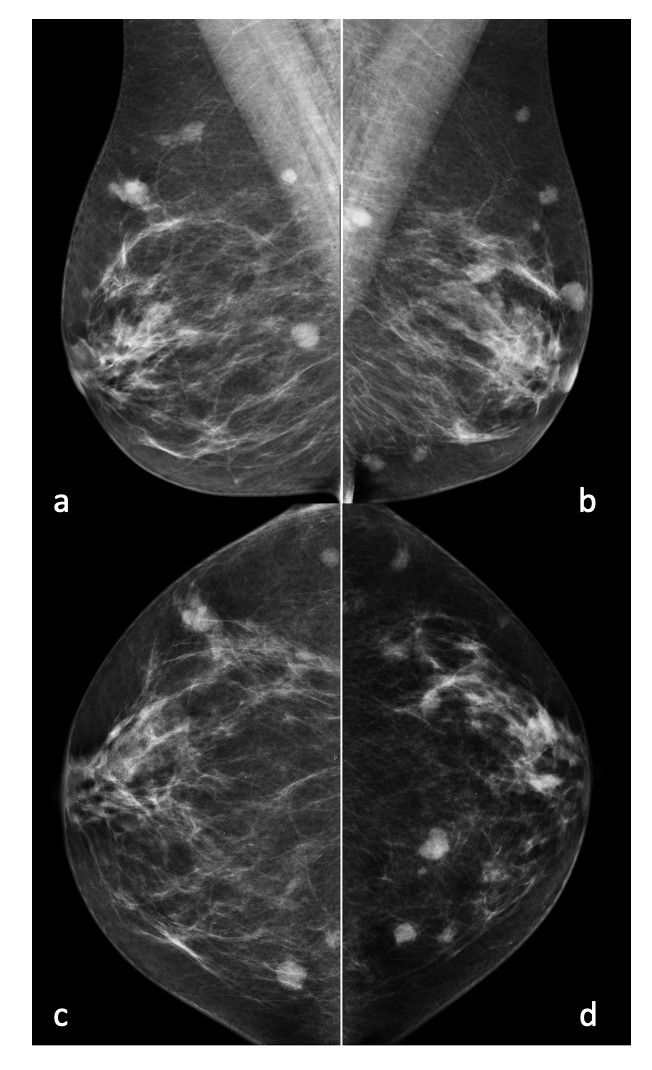
Figure 1: Mediolateral oblique (a and b) and craniocaudal (c and d) views revealing multiple oval masses with microlobulated margins in both breasts.
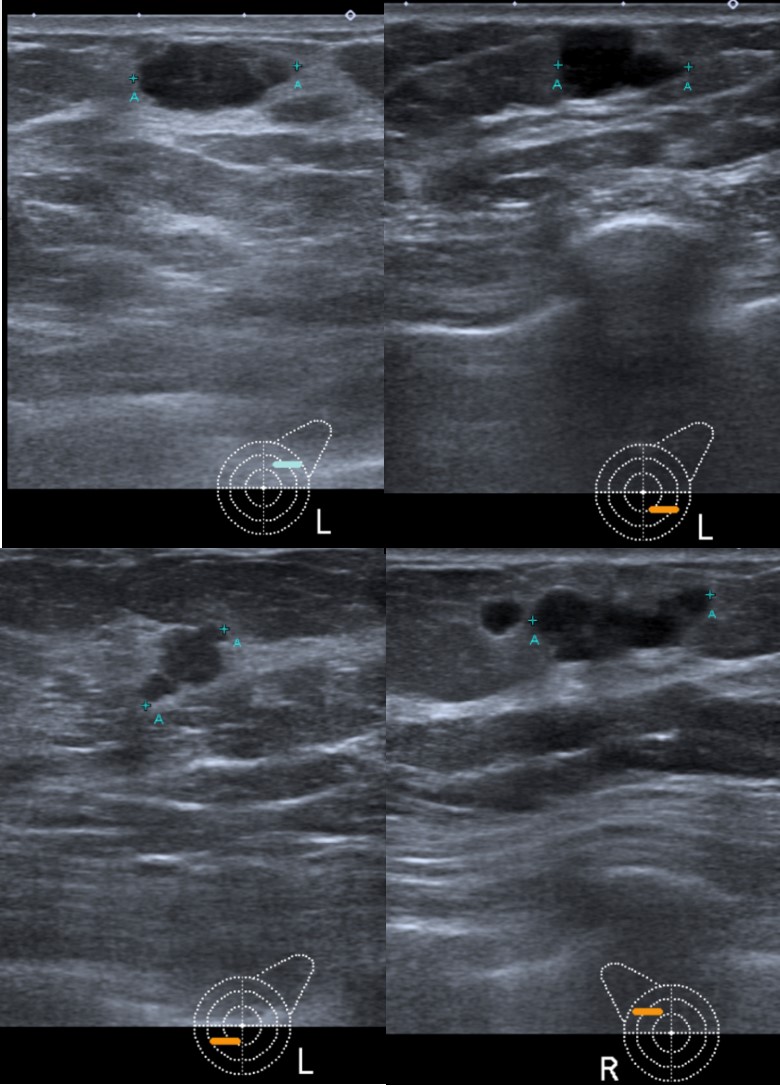
Figure 2: Gray scale ultrasonography images showed multiple hypoechoic heterogeneous masses with microlobulated margins in all quadrants of both breasts.
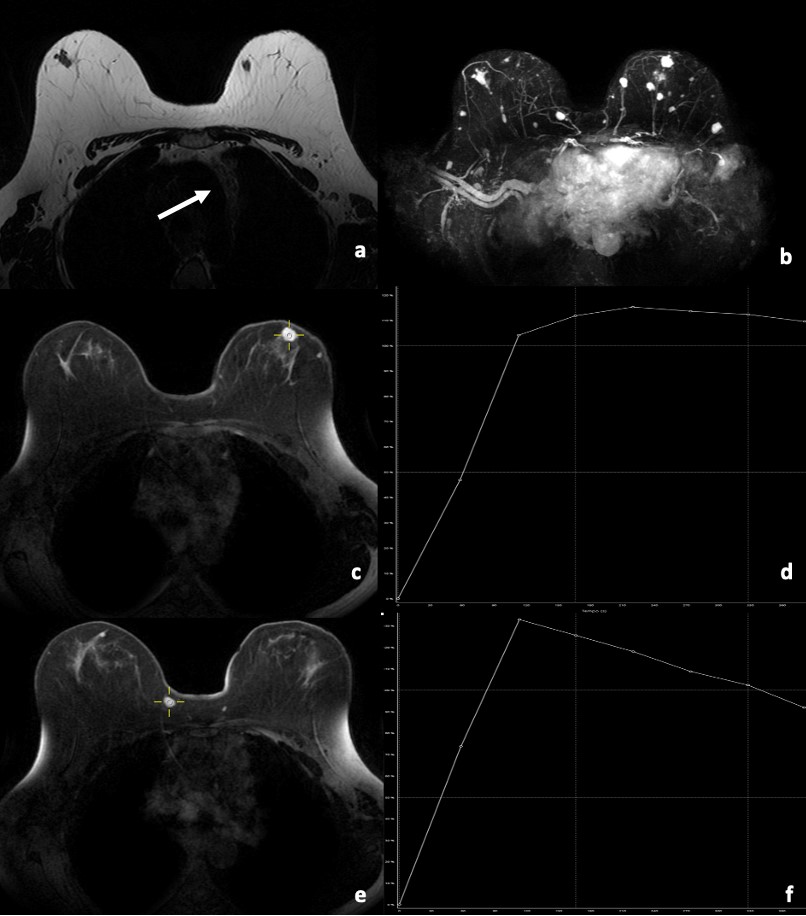
Figure 3: Axial breast MRI. (a) T2 MR-weighted image shows bilateral hypointense masses with circumscribed margins. It also shows mediastinal lymphadenopathy (arrow) (b) MIP reformatting shows multiple enhancing masses in both breasts. T1-weighted post-contrast fat-saturation images show an enhancing mass (c) with rapid initial uptake followed by a plateau phase at the kinetic curve study (d). Post-contrast T1 fat-saturated image shows another enhancing mass (e) with rapid initial uptake followed by washout phase at the kinetic curve study (f). Both curves (type 2 (d) and 3 (f), respectively) were suspicious for malignancy.
Discussion
Sarcoidosis is a chronic multisystemic inflammatory granulomatous disease of unknown etiology manifested by the presence of non-caseating granulomas that can be found in multiple organs and tissues, affecting predominantly the lungs and intrathoracic lymph nodes. Breast involvement by this inflammatory disease is rare and generally occurs in patients with well-known systemic involvement. Primary breast involvement is rare.1,2
Lesions often have a similar appearance to malignant masses, which constitute a diagnostic challenge. Diagnosis is based on clinical and radiological findings, and definite diagnosis requires histopathological confirmation with observation of non-caseating epithelioid granulomas.2,3,4
Breast sarcoidosis affects young to middle aged adults, with higher incidence in woman.5,6Clinically breast sarcoidosis presents as palpable hard masses with enlarged axillary lymph nodes, often mimicking carcinoma.2,4
Mammography, ultrasound and MRI contribute for detection of breast sarcoidosis. Since cancer is far more common than sarcoidosis of the breast, malignancy must always be considered and ruled out.3
At mammography breast sarcoidosis most often presents with irregular, ill-defined and/or spiculated masses. On ultrasound, the most common findings of breast sarcoidosis are irregular hypoechoic masses with enlarged axillary and/or intramammary lymph nodes, often considered suspicious for malignancy.2,4,5,7,8
MRI is useless for excluding malignancy in cases of breast sarcoidosis since these lesions often present as irregular enhancing masses, making it difficult to distinguish from carcinoma.3 It has also been reported that breast sarcoidosis lesions may demonstrated rapid enhancement and washout kinetic curves, rendering the distinction from cancer very difficult because these kinetics are very similar to those observed in cases of breast carcinoma.4 Sarcoidosis lesions can also show gradually enhancement over time followed by a plateau phase. Therefore, MRI has not a higher accuracy than mammography or ultrasound in excluding malignancy.5
The differential diagnosis of an irregular spiculated mass include malignancy, radial scar, fibrosis, chronic idiopathic granulomatous mastitis, foreign body reactions and fungal or tuberculosis infections. All these pathologies should be excluded.2,3,5,9Consequently, tissue core biopsy confirmation is required to establish a definitive and accurate diagnosis, allowing to differentiate breast sarcoidosis from carcinoma or from other immunological and infectious diseases.3,5,10In presence of granulomas, it is important to recognize other granulomatous disorders, such tuberculosis, Wegener’s granulomatosis, or idiopathic granulomatous disease since therapeutic approaches differ.
The definitive diagnosis is based on radiological manifestations, histological confirmation, and assessment of high serum angiotensin-converting enzyme (ACE) levels, which is a specific test used to confirm the diagnosis of primary sarcoidosis.9,11The previous diagnosis of sarcoidosis does not exclude malignancy in breast lesions.
Corticotherapy is considered the first line of treatment for sarcoidosis.10,12
In conclusion, breast sarcoidosis involvement represents an unusual manifestation of this systemic disease, with imaging features mimicking malignancy in all imaging modalities. Patients with known sarcoidosis of the breast with chronic masses, must always undergo core biopsy when presenting with a newly suspicious mass in the breast or axilla to exclude malignant lesions.