A 19-year-old female was referred to our institution for an incidental finding during a check-up ultrasound. The ultrasound showed a mixed lesion at the pancreas tail, with cystic and solid areas. The patient was asymptomatic, with no relevant laboratory anomalies and the past medical history was unremarkable.
A Magnetic Resonance (MR) was performed for further evaluation, which identified a 4 cm complex pancreatic tail lesion, exhibiting both cystic and solid components. The lesion was round and well-circumscribed, presenting a heterogeneous internal structure (Fig 1A). On T1-weighted imaging, the lesion demonstrated an intrinsically high signal, attributed to hemorrhage (Fig 1B). It was hyperintense on T2-weighted imaging and exhibited restriction on Diffusion-weighted imaging (Fig 1C, 1D). The dynamic study showed solid areas displaying a smudgy and progressive enhancement (Fig 1E, 1F). The main pancreatic duct (MPD) was normal.
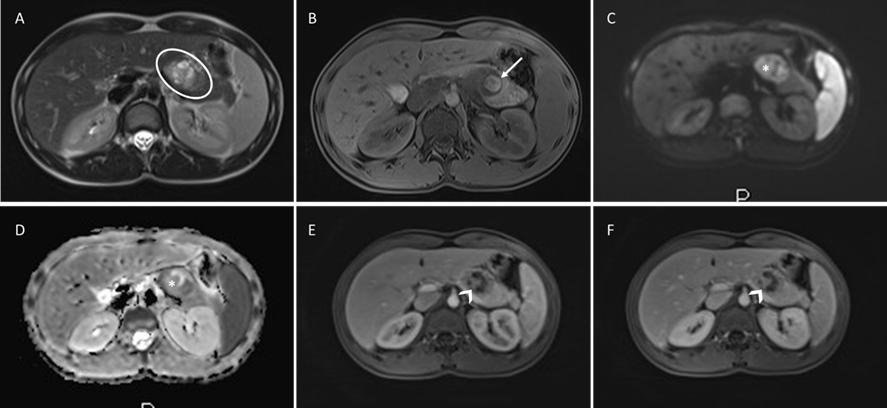
Figure 1: Solid pseudopapillary tumor of the pancreas: Round and well-circumscribed complex pancreatic lesion (circle), heterogeneous and predominantly hyperintense on Axial T2-weighted MR image(A); There is a hyperintense area (arrow) on unenhanced fat-suppressed T1-weighted MR image (B), representing blood products from tumor degeneration; on Diffusion-weighted imaging, solid areas show restriction (asterisk), being hyperintense on b800(C) and hypointense ADC map(D); Post-contrast fat-surpassed T1-weighted MR images show non-enhancing cystic areas, and the solid components (arrowhead) present mildly early in the arterial-phase(E) and progressive enhancement in the delayed-phase(F).
Subsequently, the patient was submitted to surgery and the pathological diagnosis revealed a Solid Pseudopapillary Neoplasm (SPN). The patient did not receive chemotherapy or radiotherapy after surgery. After 9 months of follow-up, the patient is still free from recurrence or metastatic disease.
SPN is a rare pancreatic tumor predominantly found in young women, by the 2nd-3rd decade.1 The majority of patients with SPN are asymptomatic, and the tumor tends to have low malignant potential, with more than 80% being benign.2 It can be present in any segment but is more frequently in the tail.3 SPN has pseudopapillary fronds with delicate vascular channels that bleed as they grow, creating cystic areas due to hemorrhage, and they may present calcification.1
SPN typically presents as a round well-encapsulated mass, exhibiting a cystic appearance due to the presence of hemorrhagic and necrotic material.1,2,3 On MRI, SPN displays a heterogeneous mildly increased T2 signal intensity. The internal hemorrhage appears with high signal intensity on T1-weighted images. The solid component of SPNs typically exhibits a smudgy early enhancement, with slow progression in the subsequent phases.1,3 Endoscopic ultrasound with fine-needle aspiration (EUS-FNA) biopsy can be useful in doubtful imaging appearances.4,5
SPN requires surgical removal due to their risk of malignancy.5,6Despite this, SPN is associated with a favorable prognosis, boasting a 5-year survival rate ranging from 71.1% to 97.0%.3 However, there is a reported recurrence or metastasis rate of 4.4% - 9.0% post-surgery, with the liver being the most common site for metastases, followed by rare occurrences in lymph nodes, peritoneum, and lungs.1
Cystic pancreatic lesions are relatively common. Despite their prevalence, the diagnosis poses challenges due to the absence of specific clinical and laboratory signs, and the overlap of imaging findings.3 In a pancreatic complex lesion, with both cystic and solid features, the primary differentials include mainly Solid Pseudopapillary Neoplasm (SPN) and Cystic Neuroendocrine Tumors (NET). True cystic lesions can also present enhancing areas, like in Serous Cystadenomas (SCA), Mucinous Cystic Neoplasms (MCN), and Intraductal Papillary Mucinous Neoplasms (IPMN). There are other solid pancreatic neoplasms that may present cystic component or degeneration, like adenocarcinoma (ADC) or metastasis.7
Cystic NET is infrequent and typically occurs in the 5th - 6th decade of life.3 This tumor is often associated with multiple endocrine neoplasia syndromes.1 Notably, due to its rich blood supply, it exhibits an intense early enhancement of the wall, which is considered a highly suggestive radiologic characteristic and aids in differentiating from SPN. The internal cystic component of Cystic NET can also be varied in appearance, but generally demonstrates low signal intensity on T1-weighted images.2
MCNs are seen nearly exclusively in women. They typically manifest as unilocular or mildly septate macrocystic lesions, with potential internal features such as necrosis and hemorrhage.1 Mucinous cystadenocarcinoma manifests as a large complex cystic pancreatic lesion, presenting papillary vegetation, nodules or T1 hyperintensity. So, it can overlap SPN, but in those cases, both conditions have indications for surgical excision.2
SCA typically exhibits a distinct imaging appearance, characterized by a classic microcystic or "honeycomb" structure, and lobulated contours.1 It is more common in the head, unlike the case presented. While its septa can show enhancement, it may rarely present as a pseudosolid variant (2%). SCA may also present with internal hemorrhage. However, the ovarian stromal origin of SCA results in early avid enhancement, similar to other hypervascular lesions (NET or metastasis), differentiating from SPN.3
Additionally, when approaching a pancreatic cystic lesion in women, epidemiology plays a crucial role with age presentation. SPN appears in the 2nd - 3rd decade ('daughter' lesion), MCN in the 4th - 5th decade ('mother' lesion), and SCA in the 5th - 7th decade ('grandma' lesion).1
IPMNs can be excluded by the absence of duct connection and the normal appearance of MPD.3 Walled-off necrosis also can have hemorrhage and necrosis, presenting as internal debris, and their enhancing wall can simulate solid component, although it only appears in a post-pancreatitis setting.3 ADC with cystic component manifests with an infiltrative pattern, vascular invasion, and ductal obstruction, facilitating differentiation from SPN.2
In conclusion, in the imaging evaluation of a complex pancreatic lesion (solid-cystic), SPN characteristic features will allow its diagnosis and differentiation from other entities. However, we highlight that malignant MCNs, like Mucinous cystadenocarcinoma, and some presentations of cystic NET can overlap with SPN’s imaging features.1,2,3 Nevertheless, all these entities require surgical resection because of their malignancy risk,4,5,6 and their preoperative differentiation may not be crucial in the clinical scenario.















