Introduction
Acute osteomyelitis (AO) is a bacterial infection of the bone that can evolve to its destruction. Paediatric prevalence has remained stable over the last decades, with an estimated rate of 1/5000 under 13 years old.1 Its global incidence is 0.2-1.6/1000, varying significantly across the world.2 About 50% of cases occur under five years of age due to the richer metaphyseal blood supply and immature immune system.1,3-5 Boys are 1.5-2 times more likely to be affected than girls.1,3,6,7
Bacteremia is the most common pathophysiologic mechanism. One-third of the patients have a history of recent trauma.1,6 Some risk factors associated with AO are sickle cell disease, diabetes mellitus, chronic renal disease, varicella infection and immunodeficiency.1,6 However, most of the cases occur in previously healthy individuals.
Long tubular bones are affected in more than 80% of AO, particularly the femur and the tibia.4,8 Bacteria reach the bone through the nutrient artery and lodge and multiply at the metaphyseal capillaries’ loops, favored by its turbulent flow.3,4 The most affected nontubular bones are the calcaneus and pelvic region.8
The usual responsible microorganisms are oropharynx colonizers, namely Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes), with an age-dependent relative frequency.3Kingella kingae (K. kingae) plays a major role in children under four years old.3,5,9 In a recent systematic review, Maria Wong et al10 found a 30.8% prevalence of K. kingae osteoarticular infections in the paediatric population, with a significant increase under 48 months of age (47.6%).10 Considering children with hemoglobinopathies SS or SC and those in developing countries, Salmonella species are an important cause of AO.3,5,7,11 Neonatal AO has a broader group of potential causative microorganisms, such as Streptococcus agalactiae, coagulase-negative Staphylococci and Enterobacteriaceae.3,5
The hallmark of AO is localized pain and impaired function.5 Diagnosis depends on clinical history, physical examination, laboratory tests, imaging, and microorganism isolation from the site of infection or blood.3
The objective of this study was to describe and characterize AO cases admitted to the pediatric ward of a second-level hospital in a six-year period (2014-2019).
Methods
A retrospective single-center study was conducted, including all the children under 18 years old with a primary diagnosis of AO admitted to our hospital. Clinical information was obtained by consulting medical records. Free and informed consent was signed by parents or legal representative. Descriptive statistics analysis was performed using Microsoft® Excel 2018.
Results
Ten AO cases were identified, six boys and four girls. The median age was 6.7 years ENT#091;1.7-13ENT#093;. Forty percent were younger than five years old and all were previously healthy. A recent history of a direct trauma was reported by 50% of the patients, which occurred 8.4 ± 11.3 days before admission. A dog bite to the hand from an adequately vaccinated family dog was reported, with no microbiological agent identified.
Local pain was referred by all the patients and impaired function or refusal to walk by nine (100% if lower limb affected). Three presented with local swelling and one with erythema. Four children had fever and only two of those exhibited the signs of inflammation mentioned above. The lower limbs were the most affected (80%): foot (n=3), tibia (n=3) and femur (n=2). The hand and the sacrum were implicated in one patient each.
Only two patients had the diagnosis considered in the first visit to the emergency department (ED), while in the majority it was admitted at the second ED visit (n=6) and two at the third. AO was confirmed after 12.5 ± 12 days ENT#091;2-32ENT#093; of symptom onset. After admission, apyrexy was achieved on day 2.4 ± 1 ENT#091;1-4ENT#093;. Resolution of local signs of inflammation and impaired function were seen on day 7.1 ± 2.3 ENT#091;3-13ENT#093;.
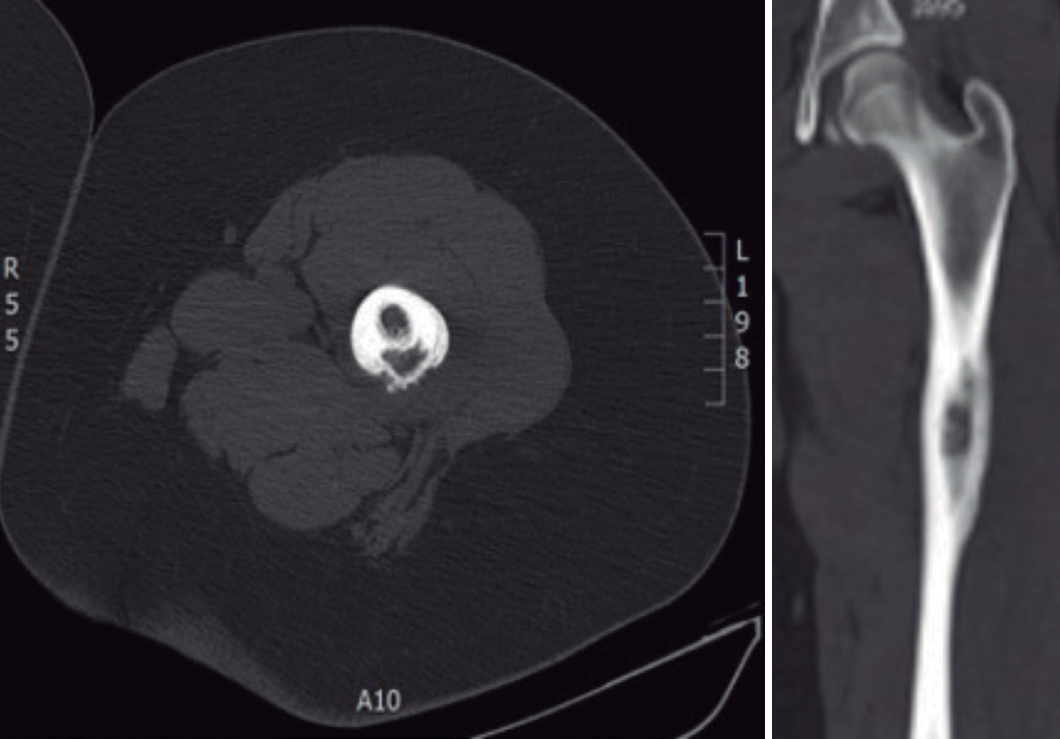
Eight patients were submitted to a radiography of the affected location at admission and only two showed alterations. Eight performed a magnetic resonance imaging (MRI), seven of which presented suggestive signs of AO (Fig. 1). Three of the five patients who were submitted to computerized tomography (CT) scan exhibited AO features (Fig. 2). Two patients performed bone scintigraphy, which showed abnormalities consistent with AO (Fig. 3).
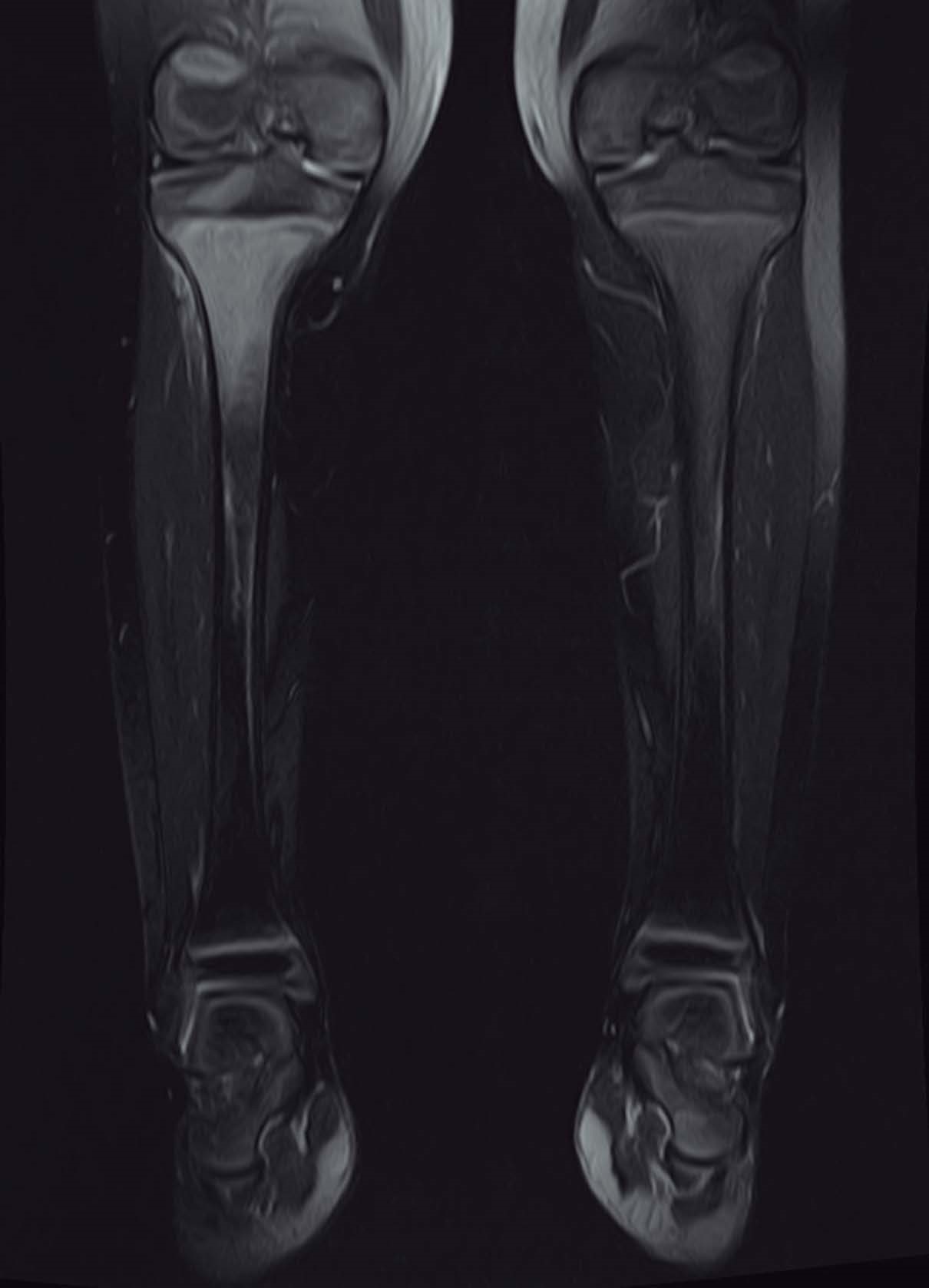
Figure 1: Coronal MRI T1-weithed shows a high signal intensity in the proximal right tibia with diffuse low-signal consistent with edema of surrounding soft tissues.
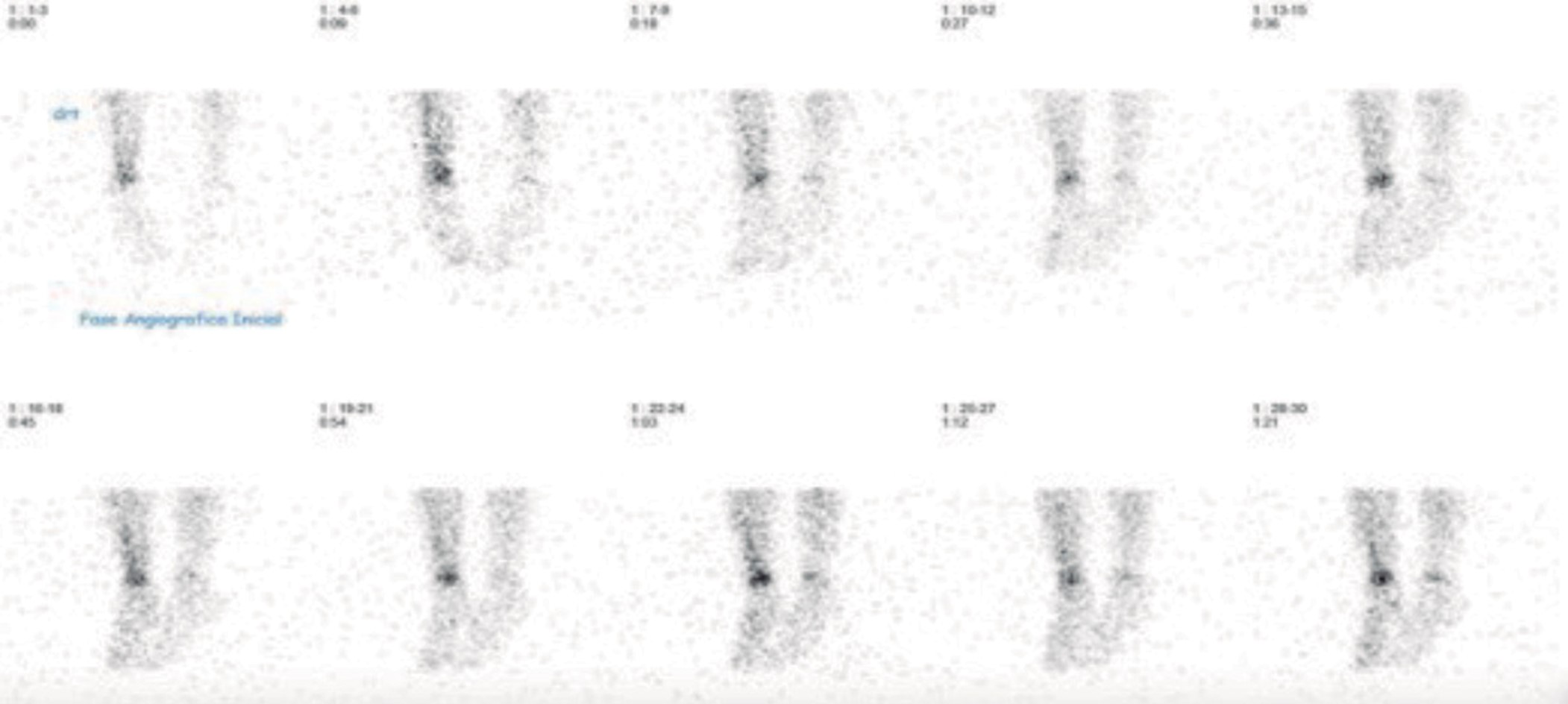
Figure 3: Scintigraphy - Increased osteoblastic activity resulting in increased levels of radiotracer uptake in the distal extremity of right tibia consistent with the osteoarticular infection suspection.
At admission, white blood cell count (WBC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were evaluated in all patients. Mean WBC was 10880 ± 3421/μL ENT#091;6900-18100ENT#093;. Five had WBC of 12000/μL or less. Mean CRP level was 4.57 ± 3.7 mg/dL ENT#091;0.5-11ENT#093; and two had a negative CRP (<0.5 mg/dL). Eight had a high ESR level at admission (>20 mm/hour) with a mean value of 41.7 ± 23.9 mm/hour ENT#091;4-85ENT#093;. During the first six to ten days after admission, ESR levels decreased in all but one and normalized in three (<20 mm/hour). All presented a drop in CRP levels after the first six to ten days, reaching normal ranges in three (Table 1).
Table 1: Evolution of inflammatory markers in the first week of after the diagnosis (6-10 days).
| Patient | At admission | Evolution | |||
|---|---|---|---|---|---|
| CRP (mg/dL) | ESR (mm/hour) | Days later | CRP (mg/dL) | ESR (mm/hour) | |
| 1 | 0.5 | 4 | 7 | 0.5 | - |
| 2 | 3.67 | 69 | - | - | - |
| 3 | 2.23 | 11 | 7 | 1.21 | - |
| 4 | 2.26 | 35 | 8 | 0.5 | 8 |
| 5 | 7.88 | 35 | 10 | 0.5 | 12 |
| 6 | 5.37 | 30 | 6 | 2.22 | 15 |
| 7 | 2.07 | 38 | 9 | 0.5 | 38 |
| 8 | 11 | 63 | 7 | 0.68 | 43 |
| 9 | 0.5 | 85 | 7 | 0.5 | 68 |
| 10 | 10.22 | 47 | 7 | 3.38 | 54 |
Blood cultures were performed in all patients with a rate of 40% positivity, identifying S. aureus (n=3) and S. pyogenes (n=1). Antimicrobial susceptibility tests didn’t detect methicillin-resistant S. aureus (MRSA). Only one patient was submitted to surgical drainage. Salmonella enteritidis was isolated from a bone abscess. A PCR test from an oropharyngeal swab was performed in a 20-month-old child, with identification of K. kingae. Considering the negative cultures for other microorganisms, a K. kingae AO was assumed in this case.
Empiric parenteral antibiotherapy was initiated with flucloxacillin in most of the cases (n=7). Other chosen antibiotics were ceftriaxone (n=2), cefuroxime (n=2), clindamycin (n=3) and gentamicin (n=1). Parenteral treatment was performed for 14.7 ± 6.2 days ENT#091;8-20ENT#093;. Patients were discharged 17.1 ± 8.2 days after admission. Oral antibiotics were flucloxacillin (n=6), amoxicillin and clavulanic acid (n=2), amoxicillin (n=1) and cefuroxime (n=1), taken for an average of 3.9 ± 0.9 weeks ENT#091;2-5ENT#093;.
The follow-up period was 8.3 ± 5.2 months ENT#091;1-17ENT#093; in eight patients. Two had several follow-up orthopedic appointments for more than one year until the present date. Two cases evolved to local complications - tenosynovitis and bone abscess requiring surgical drainage -, and one progressed to chronic recurrent multifocal osteomyelitis. Only the patient who exhibited tenosynovitis had already completed follow-up.
Discussion
AO was more prevalent in boys and the lower limb was the most affected, as described in the literature.1,6,8 A significant percentage involved children under five years of age. In the majority the cases, AO was only considered after a second visit to the ED, reinforcing its diagnostic challenge.12 Symptom onset is often insidious and clinical manifestations may be scarce.3,6,8 The classic triad of fever, pain and signs of inflammation is frequently absent and lack of specific clinical, radiological and laboratorial features delays diagnosis and treatment.12 The clinical presentation of AO is typically local pain, reported by all patients in our study; and reduced function of the affected limb or structure, which was also in accordance with our reports. In fact, all limb affected patients presented with limping or inability to walk. Other signs of inflammation such as erythema and edema were seen in less than half.
Despite the mentioned risk factors potentially related to AO, all the children were previously healthy. Bacteria can enter the bone by hematogenous route from a distant source of infection, contiguous spread from surrounding tissue and joints or direct inoculation from trauma or surgery.13 Direct recent trauma was reported by 50%, in accordance with the literature. Nevertheless, due to a high incidence of trauma in the pediatric population, its association with AO is questionable.12 In the child bitten by a dog, a direct microorganism inoculation is probable. No other possible risk factors were identified.
At admission, a radiography should always be performed to exclude other bone lesions, namely fractures or tumors.3,5,6,8 Bone destruction is not apparent in plain radiographs until the seventh to tenth day of infection,8,12,14 the time required to a sustained demineralization by inflammatory osteoclastic activity.5 In this study, AO diagnostic imaging was achieved on average by the 12th day after disease onset, and as expected, most radiographs were normal at admission. A CT scan was performed in five patients and was unremarkable in two of them. CT may be useful in identifying bone destruction and soft tissue assessment, but the gold standard is the MRI scan, which enabled the diagnosis in almost all of our patients.1,3,4,6 Initial MRI changes can be present within two to five days after disease onset and it also allows the identification of extraosseous manifestations or complications.1,3 Prior to the emergence of MRI, scintigraphy was the recommended imaging study.15 Leonard P Connollyet al concluded that this imaging strategy remains highly effective, especially if symptoms are poorly localized or occult pelvic AO is suspected.15 Scintigraphy was done in two patients and led to the diagnosis in one of them, after unremarkable radiograph and MRI scan. The other scintigraphy was performed to complete the study of a sacrum AO.
Before antibiotic therapy, blood cultures and, if indicated, bone biopsy/joint fluid samples and antibiotic sensitivity tests should always be performed.3,6 According to the literature, blood cultures are positive in 10%-40% of the cases and bone biopsy/joint fluid cultures in approximately 50%.7 Our rate of blood culture positivity 40%, similar to what is described.3 MRSA has become common in many countries, covering for 30%-40% of osteoarticular infections in children according to a United States study.3 Nevertheless, none has been identified in our population. There is lack of data concerning its prevalence in Portugal. Other AO agents include S. pyogenes, especially as a complication of varicella infection. Recent studies showed K. kingae to be an important causative agent under four years of age.9,10 Samara et al found this pathogen to be responsible for 51% of osteoarticular infections in Geneva’s pediatric population after extensive use of nucleic acid amplification assays.9 Before that, in their cohort of 129 children with AO, a microorganism identification was possible in 76 cases (59.4%), with only one K. kingae. It is important to highlight that a significant statistical difference was only identified in the group of 6 to 48 months. More recently, a Morocco’s study revealed that K. kingae was the leading cause (58.7%) of culture-negative AO in children under 10 years old, mainly between 6 months and 5 years old.16 AO caused by this agent tends to have a more benign clinical evolution, presenting with fever in less than 15% and normal CRP levels in 39%, due to its mild clinical and biological inflammatory response to infection.3,12 Invasive K. kingae disease has been related to herpes simplex stomatitis, hand-foot-mouth disease and varicella and human rhinovirus infections. The importance of the PCR assay in identification of fastidious species, such as K. kingae, should be emphasized. In a highly suspected AO case with negative conventional cultures, PCR-positive oropharyngeal swabs have a positive predictive value of 91% for detection of K. kingae.9,10 In our study, a presumptive diagnosis was assumed in an afebrile 20-month-old with a negative PCR level. Salmonella is rare cause of AO in the absence of risk factors, mainly hemoglobinopathies. In a previously healthy seven-year-old with a complicated AO, Salmonella enteritidis was isolated from pus. No history of recent gastrointestinal infection was reported. After culture collection, empiric broad-spectrum parenteral antibiotherapy is warranted.5 Antibiotic choice is determined by the most likely involved pathogens taking into consideration multiple factors, including: child’s age and comorbidities, microorganism prevalence in the community, regional bacterial susceptibility patterns and circumstances around infection onset.8S. aureus is the most frequent pathogen responsible for AO in older children so, in regions where MRSA has not emerged, semisynthetic penicillin, as flucloxacillin, or first generation cephalosporins are recommended.5 As described earlier, K. kingae should always be considered in children under four years-old, particularly if with insidious onset. There is lack of consensus regarding specific treatment of K. kingae AO. Some authors suggest amoxicillin with clavulanic acid or cefazolin while others indicate ceftriaxone or cefuroxime.17,18K. kingae is generally susceptible to beta-lactams alone, but the minimal inhibitory concentrations of these antibiotics are elevated.19 Treatment of neonatal AO should have a broader antibiotic spectrum due to the increased probability of gram-negative bacillary infection.5 In our study, flucloxacillin was the main choice.
On average, parenteral treatment was taken for 14.7 days, followed by 3.9 weeks of oral antibiotic therapy. Recently, a significant reduction in antibiotherapy duration has been proposed, mainly by shortening the initial parenteral phase to less than a week in uncomplicated cases with clinical and laboratorial improvement and typical causative agents.6,20,21 Recommendations advocate a total antibiotic therapy duration of at least four weeks in healthy children.5,8 Nevertheless, in the neonatal population, complicated cases or those caused by unusual or resistant microorganisms, such as Salmonella, a longer regimen is recommended.21
Complications are more frequently observed in MRSA infections when compared to other organisms5 and generally result from invasion of surrounding tissues, as in arthritis, tenosynovitis, subperiosteal and intraosseous abscess; or sequestrum.1,5,6,8 Surgical approach is indicated in those cases.5 More severe complications include deep vein thrombosis, septic pulmonary emboli and disseminated infection with multiorgan failure.5 Two patients presented with local complications, namely tenosynovitis and a bone abscess, that was submitted to surgical drainage.
Due to AO lack of specificity regarding clinical presentation, as well as laboratorial and radiological features, a high index of suspicion and close monitoring of clinical evolution are essential to establish an early diagnosis.8
Take Home Messages
1. AO is an invasive bacterial disease preferentially affecting male children under five years-old;
2. The clinical hallmark of osteomyelitis is localized pain and impaired function;
3. A normal radiography at admission does not exclude diagnosis because of late bone destruction;
4. The gold standard for AO diagnosis is magnetic resonance imaging;
5. Empirical antibiotic therapy should be initiated as soon as possible by parenteral route and maintained for four to six weeks. The switch to oral route should be performed towards clinical and laboratory improvement.