Introduction
The finding of a bulky abdominal-pelvic mass often presents technical difficulties in its diagnosis.
The differential diagnosis is very important and imaging is the basis for patient assessment. The estimation of the origin and nature of these lesions is of fundamental importance in patient orientation, although the final diagnosis is always histological.1
The large pelvic masses (defined by some authors as having diameters > 20 cm) usually arise from the female reproductive organs. However, these masses may also arise from the gastrointestinal system, urinary system, adjacent soft tissues, peritoneum, retroperitoneum or be metastatic.
Patients with massive intra-abdominal masses present with abdominal pain or discomfort due to huge swelling. Postural hypotension, intestinal obstruction, respiratory difficulties are also possible presenting symptoms due to mass compression.
The three cases presented here aim to highlight the difficulties of diagnosis and reinforce the need for a multidisciplinary approach in these patients.
Case Reports
Case 1
A 32-year-old nulliparous patient with irrelevant medical history, who came to the ER with abdominal pain and increased abdomen volume which had started a week before.
The physical examination revealed a large abdominal mass, and the computed tomography (CT) scan showed (Fig. 1 A) a bulky solid median and supra-uterine lesion, measuring 19 cm. It was a lesion with regular contours, with a focus of recent hemorrhage, most likely a subserous myomatous nodule. It also pointed out the presence of bilateral ovarian teratomas, on the right with 4 cm and on the left with 6 cm.
The MRI identified the presence of a bulky cystic image extending to the level of the renal vessels, with a cleavage plane with both the uterus and the ovaries, probably a cyst of the mesentery with hemorrhage. No ascites, no abdominal or pelvic adenopathies where detected (Fig. 1 B). In both ovaries, there was a non-pure cystic image, with a predominance of hypersignal in T1 and hyposignal in T1 fatsat, translating lipid content, with intermediate signal in T2. They measure 54 mm on the left and 25 mm on the right with benign semiology (ADNEX MR Score 2), compatible with bilateral teratomas.
Considering that simple mesothelial cysts are rare abdominal tumors, whose preferential treatment is surgical excision, and that ovarian teratomas are generally benign tumors occurring in women of reproductive age, it was decided, after a multidisciplinary tumor board evaluation, to propose the patient for laparotomy with the intention of bilateral removal of teratomas from the ovaries and excision of any mesenteric tumor.
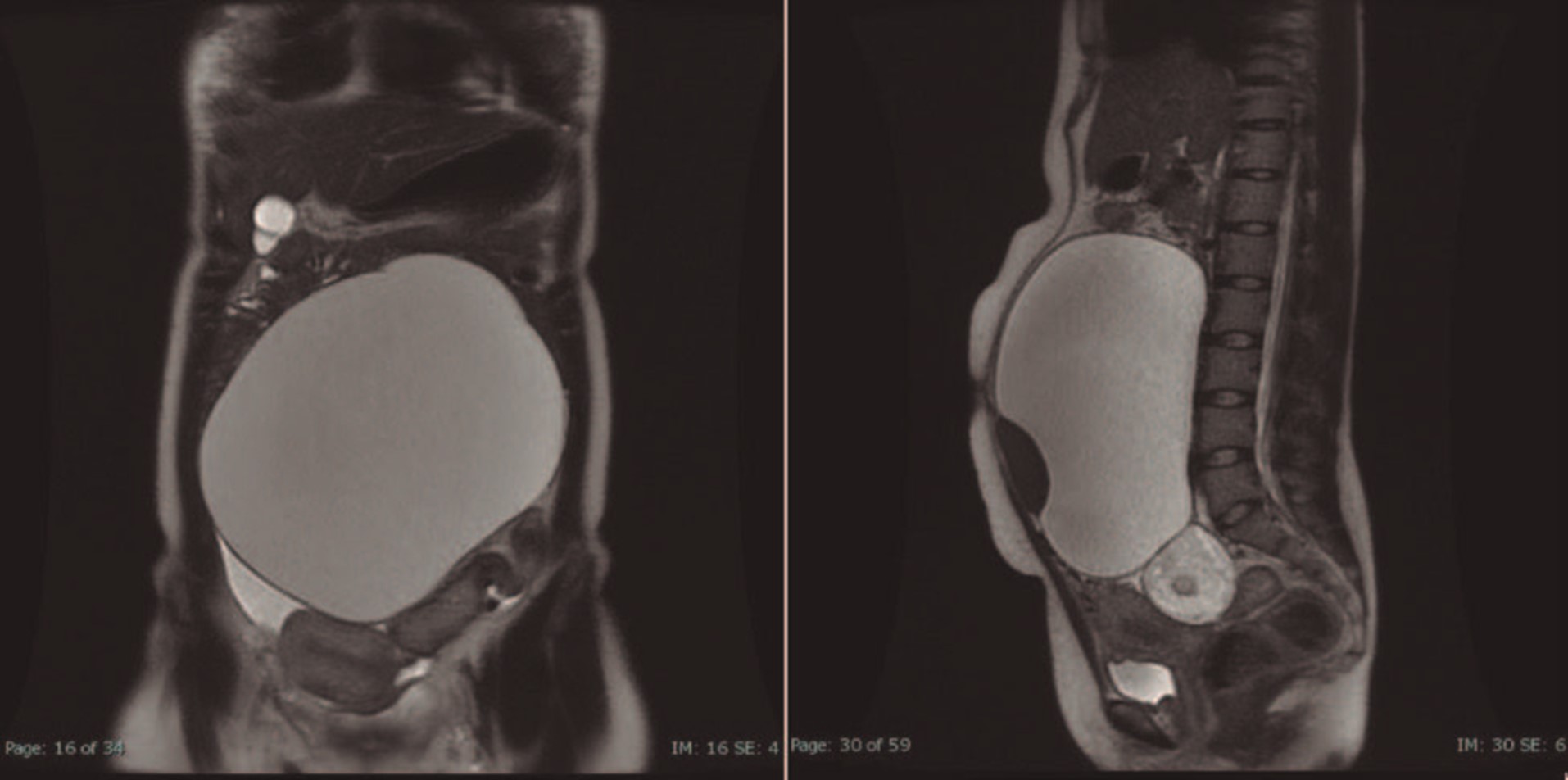
Figure 1: CT scan. The lesion was in contact with the uterus and ovary but with acute angles, which suggested an external origin.
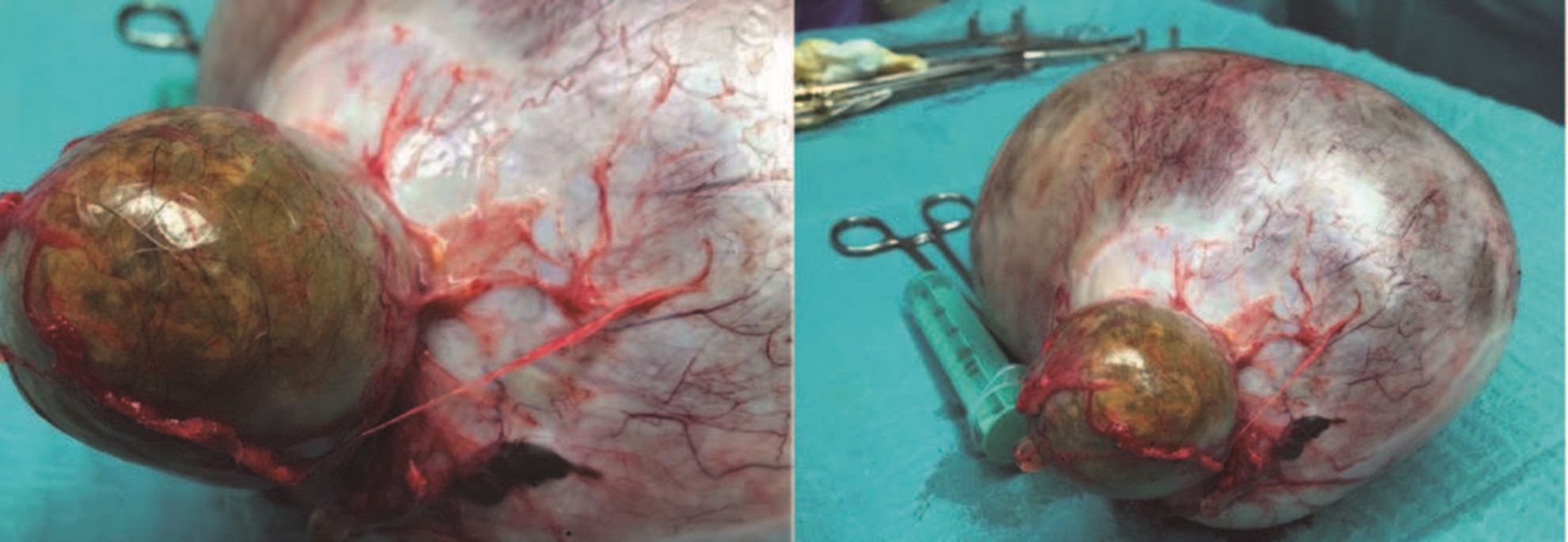
However, intraoperatively was detected the presence of a large mass coming from the left ovary of about 25 cm (Fig. 2), and a cystic formation of the right ovary of about 5 cm. Left ooforosalpingectomy and right cystectomy were performed and the diagnosis was a 3000 g bigerminal mature cystic teratoma of the left ovary, and a trigerminal cystic teratoma in the cystectomy specimen. The post-operatory period was uneventful and the patient was discharged two days after the surgery. The patient was well and had no complaints one month after and did not return to the hospital.
Case 2
A patient with 59-year-old, hypertension, menopause without hormone replacement therapy.
For several years (> 20 years) she has been complaining of meteorism, abdominal distension, changes in intestinal transit with a predominance of constipation, with some abdominal discomfort that had worsened during the last 3 months. She also mentioned episodes of heartburn without dysphagia, for a long time.
She underwent an upper endoscopy 5 years before and a colonoscopy 7 years before, which, according to patient's information, only revealed esophagitis and sigmoid diverticula.
Due to the symptoms worsening, she visited a gastroenterologist, and the physical examination revealed abdominal distention with palpation of a large abdominal mass.
A CT Scan revealed a huge abdominopelvic mass apparently with origin in the left right ovary, predominantly cystic but with septa and solid component. There were significant ascites and multiple thickening of the peritoneum and ommentum, in relation to implants on these locations (Fig. 3).
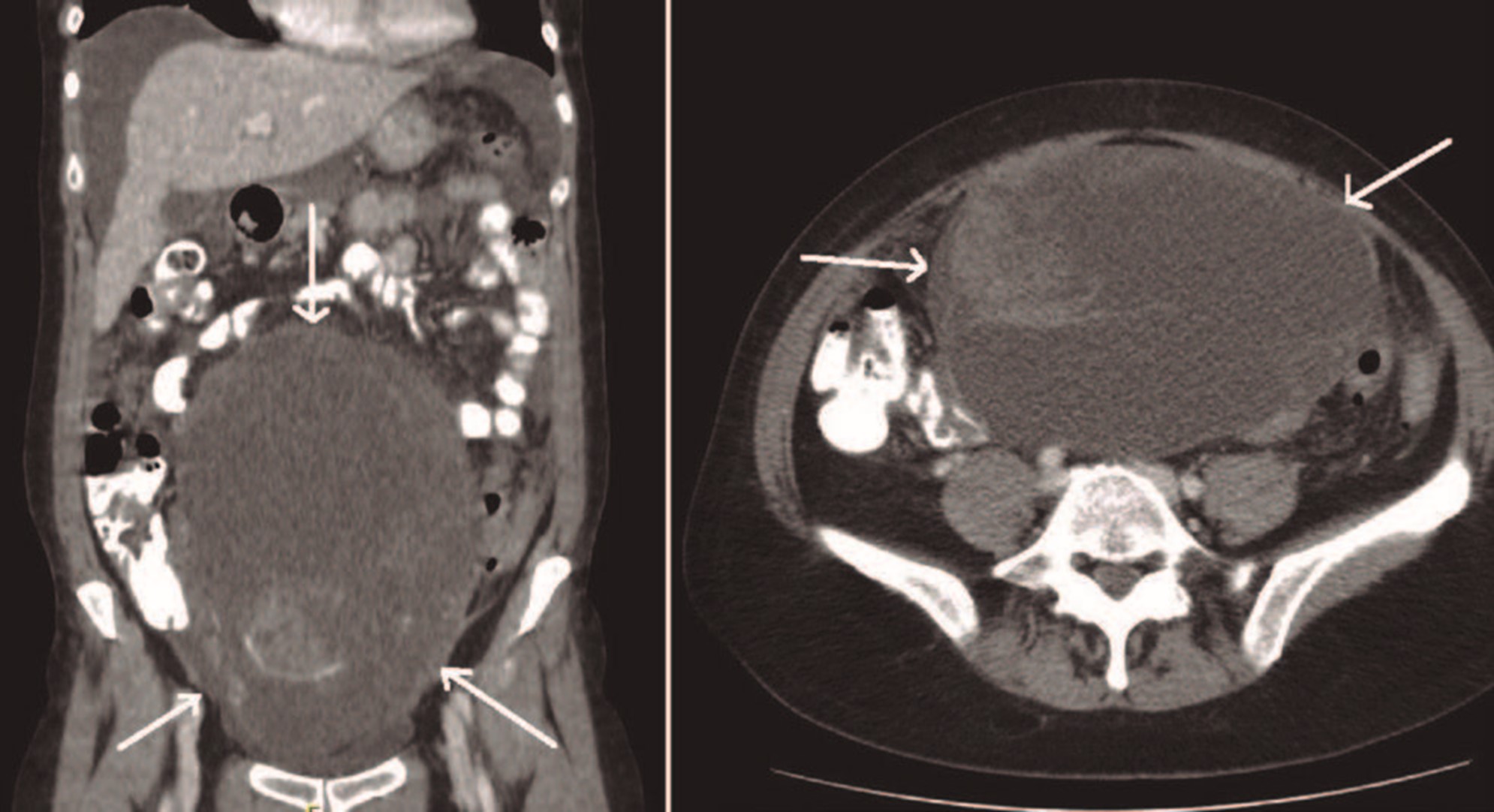
Figure 3: CT coronal and axial plan - bulky abdominopelvic mass, predominantly cystic with septa and solid component (arrows).
Blood tests revealed anemia (Hg = 9.5 g/dL) and negative tumor markers (Ca125 = 8.9 U/mL, Ca19.9 = 13 U/mL and CEA < 0.5 ng/mL).
The patient was referred to Gynecology Oncology, and during the evaluation on the multidisciplinary tumor board, doubts arose about a thickening in the transverse colon that might be the result of an omental implant, but due to its concentric character was suggestive of a primitive lesion of the colon (Fig. 4 A arrows).
The colonoscopy revealed (Fig. 4 B) a stenosis of the transverse colon with an infiltrative, insurmountable lesion, whose biopsy confirmed a well differentiated adenocarcinoma with morphology and immunohistochemical profile compatible with colorectal origin.
Surgery was performed by a gynecologist and a general surgeon. Intraoperatively, a large mass of right adnexal origin was confirmed, excised and sent for frozen section, revealing a 5000 g ovary with morphologic pattern favoring a low grade papillary serous tumor.
Due to the possibility of two synchronous tumors, it was decided to perform complete ovarian tumor cytoredution, including total hysterectomy with left ooforosalpingectomy, pelvic peritonectomy (due to the presence of implants in the posterior cul-de-sac), pelvic and lombo-aortic lymphadenectomy and a right hemicolectomy with end-to-end ileocolic anastomosis with en bloc excision of the ommentum. A complete cytoredution (CRo) was achieved.
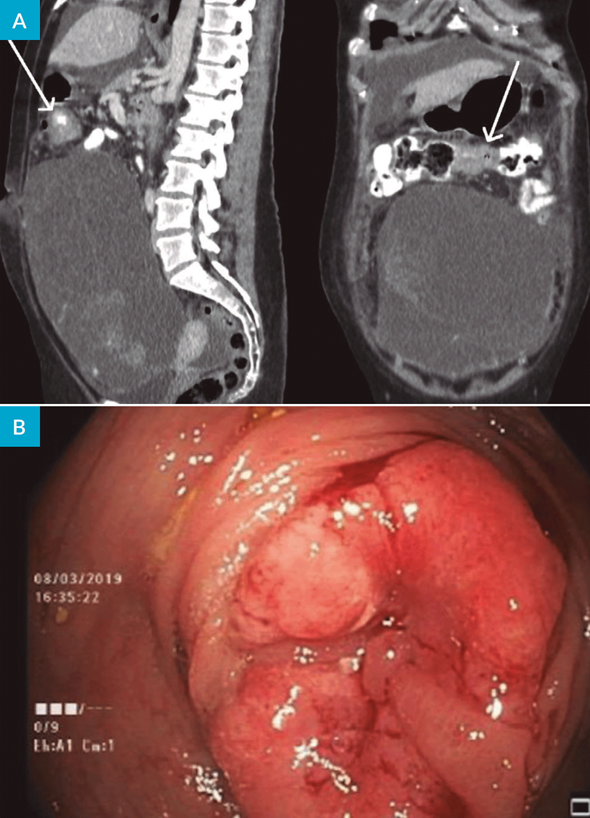
Figure 4: A - CT coronal and sagittal plan showing a concentric stenosis on transverse colon (arrow); B - Colonoscopy till the middle proximal transverse, where an infiltrative, insurmountable lesion is present.
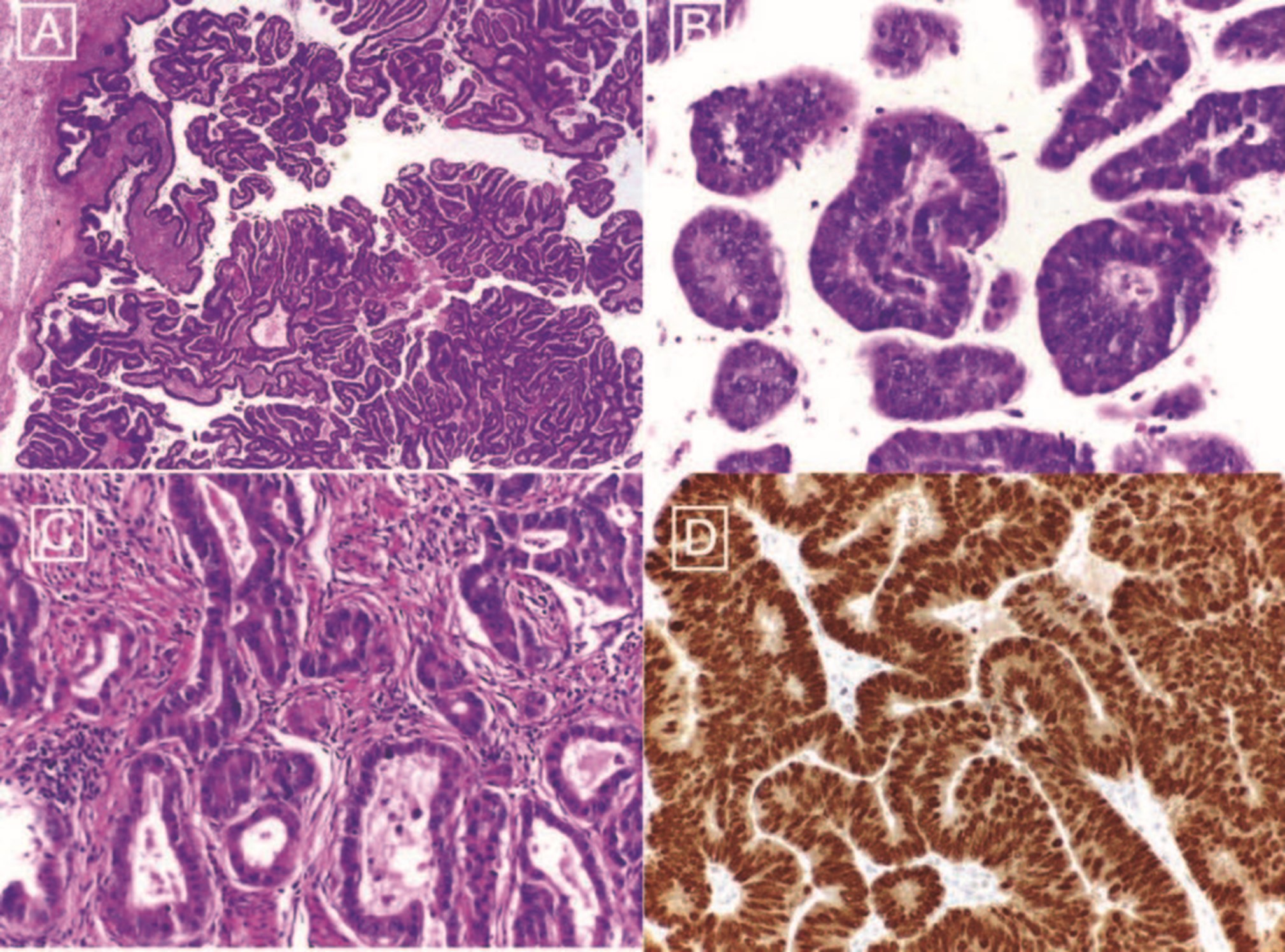
The final histological showed a moderately differentiated adenocarcinoma with solid and papillary pattern and small foci of necrosis in the right ovary. The left ovary, which was macroscopically normal, showed small foci of neoplastic tissue.
However, although the histological pattern was consistent with an ovarian tumor, the immunohistochemical study revealed strong and diffuse positivity with CDX2 and CK20 while WT1 and p53 were negative. As such, the final diagnosis was an adenocarcinoma of the right colon, G2 with metastasis in a peri-colic lymph node (1/28), on both ovaries and on pelvic peritonectomy specimen, with a phenotypic pattern consistent with colorectal origin (Fig. 5). The 41 nodes of pelvic and lumbo-aortic lymph node dissection did not show any metastasis.
The patient was referred to Medical Oncology where she started adjuvant chemotherapy and is currently well with a 2-year follow-up and no evidence of disease recurrence.
Case 3
A 67-year-old healthy woman.
For 3 days with diffuse abdominal discomfort, more pronounced in the left hypochondria, alteration on the intestinal habits with liquid diarrhea alternating with pasty stools, but without melena or rectal hemorrhage.
She described an increase in the volume of her abdomen for 3 months, which she did not find important because she had been very anxious with family problems.
Physical examination showed a distended abdomen with umbilical hernia and an abdominal mass that seemed to reach the costal grid.
A CT scan (Fig. 6) confirmed the presence of a massive lesion occupying all the abdominal and pelvic cavity, with coarse septa and some solid areas, measuring 30 cm in diameter. The presence of ascites in great quantity and a thickening of the peritoneum and the ommentum was noted in relation to probable secondary locations in these structures.
The only remarkable blood test was a Ca125 = 163.
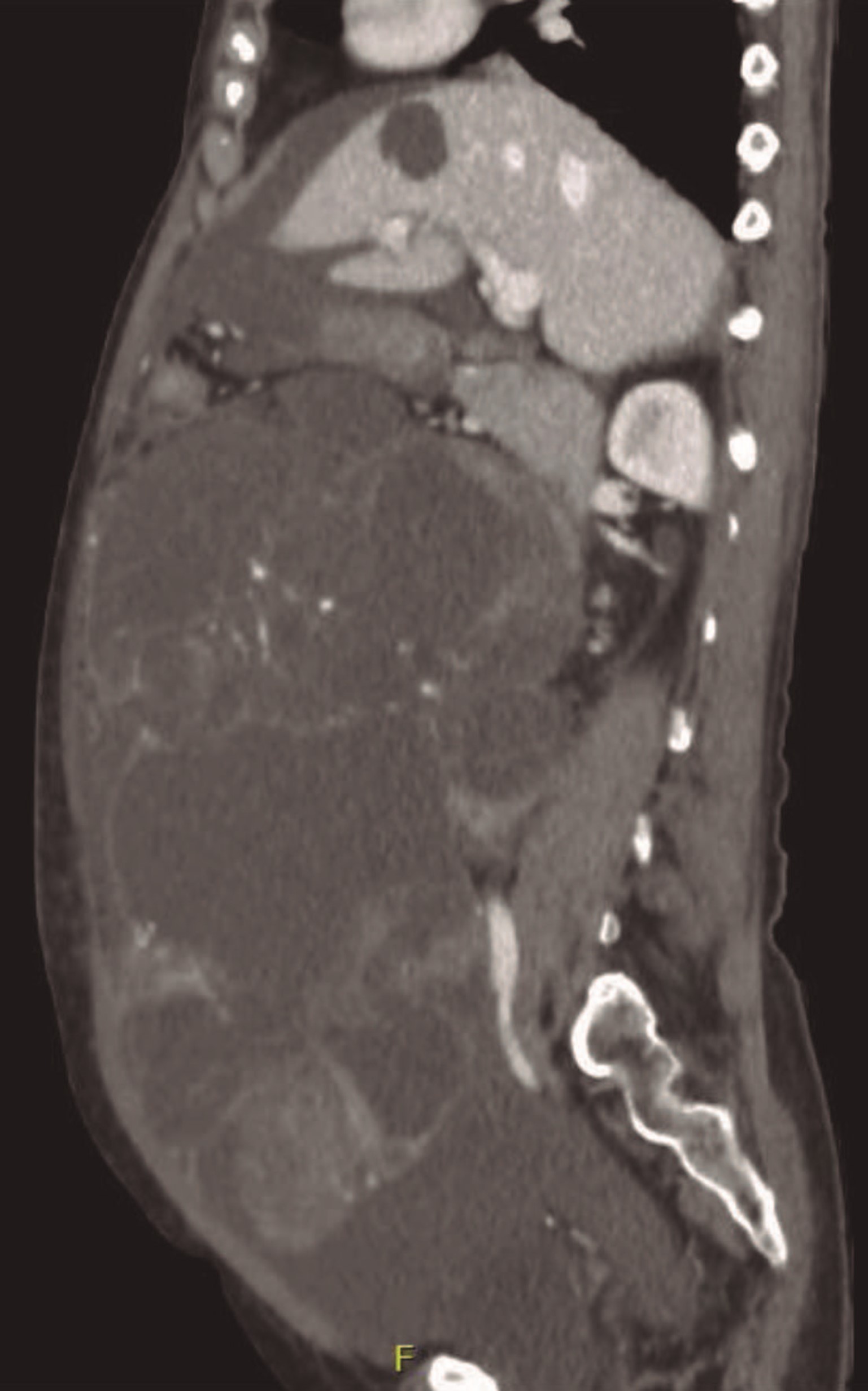
Figure 6: CT sagittal plan: huge multiloculated mass with coarse septa and solid areas suggesting an atypical lesion probably from the ovary.
Surgery was performed and the presence of a left adnexal mass was confirmed. The excision for frozen section examination revealed a mucinous borderline tumor but, due to the volume of the piece (3900 g/32 cm diameter), the final diagnosis was deferred for paraffin histology.
Final histology confirmed a borderline mucinous tumor/atypical proliferative mucinous tumor (intestinal type) with foci of intraepithelial carcinoma. No invasive or non-invasive implants were documented in the remaining parts of the surgical staging.
The patient stays well after a 3-year follow-up period.
Discussion
The etiology of pelvic masses can have gynecologic or non-gynecologic origins and ranges from benign to malignant.
In these large tumors, it may not always be possible to determine the site of origin and differential diagnosis is very important. Imaging techniques are usually diagnostic, but in some cases, they cannot give exact results.
There are some risk prediction models, IOTA SR (International Ovarian Tumors Analysis-Simple Rules), IOTA Adnex Model, but it is not the aim of this paper to discuss the tools to diagnose adnexal masses, only to highlight the difficulties in the diagnosis of very large adnexal masses.
In the first case, magnetic resonance imaging (MRI) lead to the suspicion of mesenteric cyst. The lesion was in contact with the uterus and ovary, but with acute angles, which pointed to an extrauterine origin. When the origin is in the organ, the angles are usually obtuse, but in this case, it deceived us. Very large masses also make this assessment more difficult.
Cystic ovarian teratomas, especially mature cases, constitute 10%-13% of all ovarian tumors and are the most common benign ovarian germ cell tumors. They typically occur during reproductive age.2-4
Giant ovarian teratomas commonly present with acute abdominal pain caused by adnexal torsion and abdominal distention due to the rapid growth of a large unilateral tumor, undergoing capsular distention, hemorrhage or necrosis.
The patients may also have certain non-specific abdominal complaints indicating mass effect, dyspnea and the symptoms of other organs becoming compressed by the tumor. Therefore, the aim of treatment is to reduce the severity of teratoma-related symptoms, especially to reduce the mass effect due to the raised abdominal pressure, and to prevent the potential malignancy.
Mesenteric cysts are very rare intra-abdominal benign tumors that can pose serious diagnostic and therapeutic challenges.5
The presentation of a mesenteric cyst may range from incidental asymptomatic diagnosis during radiologic procedures to non-specific symptoms such as abdominal distension, abdominal pain and abdominal bloating. This non-specific symptomatology often poses a great diagnostic challenge. Preoperative diagnosis is usually done with the use of imaging modalities being MRI the most accurate.
In our case, an MRI suggest an abdominopelvic mesenteric cyst occupying the entire midline up to approximately the level of the renal vessels, but the intraoperative view was of a large mass of about 20 cm, with multiple adhesions to the bowel and mesentery with origins on the left ovary. The presence of about 150 cc of ascitic fluid and a right enlarged ovary with a cyst 5 cm in diameter was in accordance with the MRI.
In case 2, although the morphologic aspect was in favor of a primitive ovarian cancer, this was not confirmed by the immunohistochemistry study.
Krukenberg tumor (KT) is defined by the World Health Organization as ovarian carcinoma characterized by the presence of stromal involvement, mucin-producing neoplastic signet ring cells, and ovarian stromal sarcomatoid proliferation. The term has also been applied to metastatic ovarian tumors originating from gastrointestinal adenocarcinomas. Up to 30% of ovarian malignancies are in fact metastatic tumors, with the stomach, colorectal, and breast being amongst the most common sites of origin. KT were reported in 3%-14% of women with colorectal cancer.6 The ovary is the second most common intra-abdominal solid organ site of metastasis of colorectal cancer after the liver. The presence of KT appears to indicate extensive malignant spread within the abdominal cavity. Indeed, the prognosis for KT of colorectal origin is poor and most patients die within 1 year after diagnosis of ovarian metastasis.
Attention should be paid to the ovaries of women with colorectal cancer, particularly pre-menopausal women, both at the time of surgery and during follow-up.
Immunohistochemistry is a valuable tool in differentiating primary ovarian tumors from metastatic tumors. Immunostaining profile of CK 7 + and CK 20 negative are in favor of primary ovarian carcinoma, whereas metastatic gastrointestinal carcinoma is CK 7 negative and CK 20 positive6 as in our case.
Limited treatment options and poor prognosis make KT a mysterious and fatal tumor, however as in our case timely diagnosis and correct surgical treatment of the primary site, with a complete cytoreduction (CR0) along with regular follow-up can prolong the survival time.
The presence of concomitant colorectal and ovarian cancer has been reported in many series. Earlier reports stated that the incidence of cancer in additional organs in females with ovarian cancer is 2.8% and the most frequent combinations are ovarian and lung cancer or ovarian and colon cancer.7
In case 2 our surgical option was to treat the patient as if synchronous colonic and ovarian tumors were present, and only the immunehistologic study could give us the final diagnosis.
Our patient in case 3, had a late presentation as she was under emotional and familiar stress and postponed her visit to the doctor. The newer imaging modalities have made giant abdominopelvic tumors a rarity, however occasionally such cases of giant tumors have been reported.
Serous cystoadenomas of ovaries represent 40% of all ovarian masses and are usually bilateral and multi-loculated. Most of them are benign but approximately 10% have malignant potential and 20% to 25% are malignant depending upon the presenting age of the patient.
Benign mucinous cystoadenomas usually present in giant form and are among the largest known tumors. Giant tumors of the ovaries have been reported in the literature. Spohn et al have reported an abdominal cyst of 148.6 kg.8
However malignant tumors constitute 10% to 20% of these giant ovarian masses, making it necessary to exclude its possibility in all cases of ovarian masses. Tumor markers like cancer antigen 125, carcinoembryonic antigens, human chorionic gonadotropin, alpha fetoprotein, and HE4, play an important role in early diagnosis, management and follow-up of patients.9,10
In addition, the presence of multilocular cystic lesions, thick septa, solid areas, intracystic vegetations, ascites and intra-abdominal metastases are also known to be important parameters during the evaluation of these patients, because they are indicative of malignant lesions.
In our case, fortunately, despite the elevated level of Ca125 and the suspicious signs on CT scan, the frozen section and final histology were coincident and revealed a borderline mucinous tumor.
Conclusion
The differential diagnosis of large female pelvic masses is extensive. The site of origin (uterus, cervix, adnexa, rectum, bladder, pelvic muscles), imaging characteristics and clinical history may all help narrow the diagnostic hypothesis.
The most important factors are early detection, adequate preoperative evaluation, including the risk prediction models, and subsequent surgical management with multidisciplinary approach in order to decrease intra and post-operative complications and improve patient quality of life.