Introduction
Family planning is a fundamental element of reproductive health, and counseling on this topic is a responsibility of family doctors in primary health care, who should provide complete, impartial, and scientifically correct information about all contraceptive methods.1 In Portugal, 94% of sexually active women report using a contraceptive method, the most common being the contraceptive pill (58%-75%),2,3 and frequently look for counseling regarding reproductive health and contraceptive methods in their family physician.2
According to the United Nations and the World Health Organization, reproductive health is a state of complete physical, mental, and social well-being, in all matters relating to the reproductive system and to its functions and processes.4 Thus, reproductive health implies the ability to have a satisfying and safe sexual life.4 With this in mind, contraceptive counseling should include the choice of a method to avoid pregnancy, but that also has neutral or even positive effects on the sexual function of women.5,6
Most current contraceptive methods are hormonal in action. The hormonal components of contraceptives could have various effects on mood and libido, which are not clearly understood.5,7-13 The majority of women are unaffected, but reports of sexual side effects can go up to one in five oral contraceptive pill users,10 and for the individual woman who is negatively affected, this can have a substantial impact on quality of life and relationship.14 In addition, these negative sexual side effects can lead to discontinuation of the contraceptive method and loss of the pregnancy prevention effect.10,11,15-17
Luckily, nowadays women and their health care providers are attributing greater importance to sexual health, including sexual well-being.18 As such, it is fundamental for health care providers to be aware that contraceptives can have negative effects on female sexuality so they can counsel their patients appropriately.16,19-22
This review aims at identifying the existing evidence regarding the role of the different contraceptive methods on female sexual function, thus allowing better counseling on this topic by health practitioners.
Methods
This work consists of a review of the existing evidence regarding the role of the different contraceptive methods on female sexual function. In this work, the definitions of contraception and sexual dysfunction are considered as follows.
Contraception is defined as the intentional prevention of conception through the use of various devices, sexual practices, chemicals, drugs, or surgical procedures.23 The approved contraceptive devices commercialized in Portugal are summarized in Table 1.
Sexual dysfunction is defined a disturbance in sexual desire or the sexual response cycle that cause marked distress and interpersonal difficulty.24,25 This includes disorders of desire, sexual arousal, or orgasm, as well as conditions related to pain, such as vaginismus and dyspareunia.24,25
Table 1: Contraceptives commercialized in Portugal.26
| Oral hormonal contraceptives | |||||||
|---|---|---|---|---|---|---|---|
| Ethinylestradiol | Estradiol valerate | Estradiol | No estrogen | ||||
| Progestin | 15 µg | 20 µg | 30 µg | 35 µg | |||
| Levonorgestrel | X | X | |||||
| Gestodene | X | X | X | ||||
| Desogestrel | X | X | X | ||||
| Drospirenone | X | X | X | ||||
| Dienogest | X | X | |||||
| Nomegestrol acetate | X | ||||||
| Chlormadinone acetate | X | ||||||
| Cyproterone acetate | X | ||||||
| Vaginal hormonal contraceptives | |||||||
| Etonogestrel | X | ||||||
| Transdermal hormonal contraceptives | |||||||
| Norelgestromin | X | ||||||
| Intrauterine hormonal contraceptives | |||||||
| Levonorgestrel (13,5 mg) | X | ||||||
| Levonorgestrel (19,5 mg) | X | ||||||
| Levonorgestrel (52 mg) | X | ||||||
| Subcutaneous hormonal contraceptives | |||||||
| Etonogestrel | X | ||||||
| Intramuscular hormonal contraceptives | |||||||
| Medroxyprogesterone acetate | X | ||||||
| Non-hormonal contraceptives | |||||||
| Copper intrauterine dispositive (T 380, U 375) | |||||||
| Condoms (male and female) | |||||||
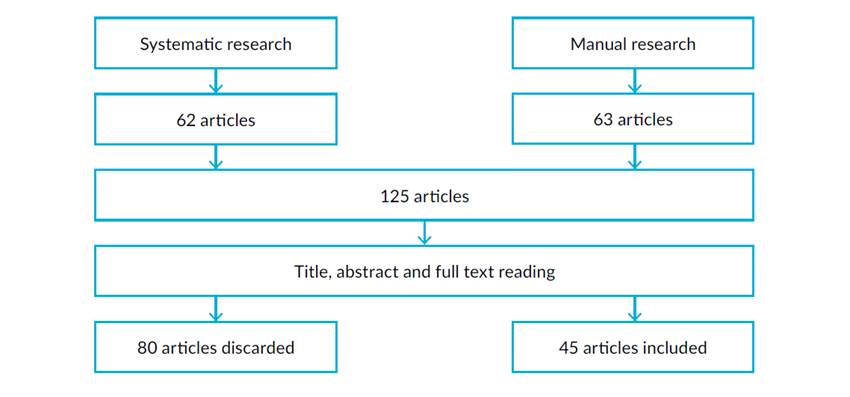
In august 2022, a systematic research of existing clinical trials, reviews, and meta-analyses, published in Portuguese or in English in the previous ten years, was performed. The research was done using PubMed.gov search engine, with the terms “(Contraceptive Agents) AND ((Libido) OR (Sexual Dysfunction) OR (Sexual Arousal) OR (Orgasm))”. The criteria for inclusion were the mention of any contraceptive method on female sexual function on any domain, including libido, arousal, pain, or others. Articles not meeting these criteria were excluded. In the research, 62 articles were found meeting the defined criteria. A direct manual research provided 63 additional articles meeting the previously defined criteria. After reading the title, abstract, and full text, 80 articles were discarded for various reasons, which included duplication, not being related to the topic of research, or not corresponding to studies in humans, leaving 45 articles that were included in this review. This is summarized in Fig. 1.
Results
This review included 45 articles on the topic of contraceptive methods and female sexual function, 13 reviews and 32 clinical trials. Of the reviews, three were systematic reviews,27-29 eight were narrative or non-systematic reviews,14,17,19-21,30-32 one was a statement from the European Society of Sexual Medicine,18 and one was a letter to the editor.33 The 32 clinical trials focused on different contraceptive methods and different outcomes, such as the Female Sexual Function Index (FSFI), the Female Sexual Distress Scale (FSDS), the McCoy Female Sexuality Questionnaire (MFSQ), and the New Sexual Satisfaction Scale (NSSS). The characteristics of the studies were fairly different, with population sizes ranging from 229 to 261213 women (median 164, IQ 269). The studies were performed in different populations and cultures, the majority (n=10; 31%) in Italy,5-7,9,34-39 six in the United States of America,10-12,40-42 three in Iran,43-45 two in Belgium,46,47 Sweden,15,22 and Egypt,48,49 and one in the Netherlands,8 Germany,13 Austria,50 Lithuania,16 South Africa,51 and Thailand.52 One study was multicenter, involving patients from Australia, Austria, Belgium, Germany, Italy, Spain, and Thailand.53
Sexuality is a fundamental part of human life and sexual dysfunction has a negative impact on both quality of life and emotional well-being.16,20,32 Female sexual dysfunction is a very common disorder, with an estimated prevalence of 22%-43% of women worldwide, with low desire being the most common.16,19,20,42 Female sexual function is believed to be multifactorial, involving biologic, physiologic, anatomic, medical, affective, interpersonal, psychological, and context-related factors.14,15,20,31,32
Contraceptive methods may be hormonal or non-hormonal, depending on their mechanism of action. Mode of usage, efficacy, and side-effects vary accordingly.26 As was previously thought, most studies report a negative impact of contraception on sexual function, specially of hormonal contraceptives.12,22,44
The impact of hormonal contraception extends in many domains of sexual function, including arousal, lubrication, pain, orgasm, pleasure, and frequency of sexual activity.12,22,41 Contraceptives may affect women’s sexuality in both positive (relief of gynecologic pain,14,18 elimination of the fear of pregnancy,7,14,18,38 increase of self-esteem in cases of acne or hirsutism,14,18 increase of intrapartner satisfaction,30 and increase in number of sexual intercourses30) and negative ways (hormonal deregulation,18 decrease of sexual desire,30 decrease of lubrication,14,30 decrease of orgasm,30 and increase of pain14). However, when controlled for other factors, such as relationship length, the impact tends to be lower or even disappear, thus meaning that contextual factors may be stronger predictors of sexual desire than contraception type.41
Up to 27% of women report a decrease in sexual drive attributed to hormonal contraceptive use.30 In a study in German female medical students, contraceptive use was shown to increase FSFI total score compared to non-users, with FSFI scores higher in non-hormonal contraceptives, followed by non-oral hormonal contraceptives and, lastly, by oral hormonal contraceptives.13 In an analysis of all contraceptive methods, the least sexual dysfunction was detected with condom use.44
Oral hormonal contraceptives and female sexual function
Two different types of contraceptives exist in an oral formulation - combined oral contraceptives and progestogen-only oral contraceptives.26 Combined oral contraceptives consist of an association of an estrogen and a progestogen. Most frequently, the estrogen component is ethinylestradiol,7,26 but more recently, estradiol valerate and estradiol have appeared, with seemingly less metabolic effects and a better safety profile.26 Progestogens differ on their androgenic or antiandrogenic activity, with levonorgestrel, gestodene, and desogestrel having a weak or neutral androgenic effect, and cyproterone acetate, dienogeste, chlormadinone acetate, nomegestrol acetate, and drospirenone having an antiandrogenic effect.7,26 Progestogen-only oral contraceptives exist as isolated desogestrel and drospirenone.26 Different regimens of administration exist, including cycles of 21 days followed by a 7-day interval/placebo, cycles of 24 days followed by a 4-day interval/placebo, or continuous administration without free intervals.26
Most data regarding the impact of contraceptives in sexual function regards oral hormonal contraceptives. The estimated prevalence of female sexual dysfunction induced by oral contraceptives ranges from 5% to 20% worldwide.32 Most reviews on the subject identified mixed results,19,29 with many studies reporting an increase or no impact in sexual desire, and less reporting a decrease in sexual desire.28,29 The studies identified in this review regarding the effects of oral hormonal contraceptives are summarized in Table 2.
Table 2: Summarized analysis of the articles included in the review regarding oral hormonal contraceptives.
| Study | Contraceptive method | Results | Conclusions |
|---|---|---|---|
| Guida et al, 201439 | Oral contraceptives | Improvement of anxiousness, sexual pleasure, frequency and intensity of orgasm, satisfaction, sexual interest, and number of sexual intercourses. | Positive impact |
| Caruso et al, 201336 | 20 µg ethinylestradiol/ 3 mg drospirenone (24-4-day cycle) | After 2 cycles, worsening of FSFI score due to a non-statistically significant reduction of all items; After 4 cycles, improvement of total FSFI score, mainly in orgasm, satisfaction, and pain. | Positive impact |
| Shahnazi et al, 201545 | 30 µg ethinylestradiol/ 150 µg levonorgestrel | Sexual functioning decreased at 2 months and increased above baseline at 4 months. | Positive impact |
| 30 µg ethinylestradiol/ 150 µg desogestrel | Sexual functioning increased at 2 and 4 months. | Positive impact | |
| Boozalis et al, 201610 | Oral contraceptives | No association with lack of libido. | No impact |
| Hassanin et al, 201849 | 30 µg ethinylestradiol/ 150 µg levonorgestrel | No significant differences. | No impact |
| 750 µg levonorgestrel | Lower total FSFI scores and in desire, arousal, lubrication, and orgasm domains. | Negative impact | |
| Zethraeus et al, 201615 | 30 μg ethinylestradiol/ 150 μg levonorgestrel | Impairment of sexual desire, arousal, and pleasure. | Negative impact |
| Čiaplinskienė et al, 201616 | 30 µg ethinylestradiol/ 3 mg drospirenone | Decline in sexual function, specifically desire and arousal. | Negative impact |
| Battaglia et al, 20129 | 30 µg ethinylestradiol/ 3 mg drospirenone | Reduction in MFSQ score, frequency of sexual intercourse, and orgasm frequency, and increase in pain during intercourse. | Negative impact |
Notes: FSFI - Female Sexual Function Index, MFSQ - McCoy Female Sexuality Questionnaire.
The reported negative effects of oral contraceptives include vaginal dryness and decreased lubrication, decreased arousal, sexual pleasure, and orgasm frequency, and increased sexual pain,18,31 sometimes related to morphological alterations of the vulvar vestibular mucosa, such as atrophy and secondary vulvar vestibulitis.18
Regarding the effect of different types of oral hormonal contraceptives, evidence is also uneven. Different doses may have different effects. A lower dose of ethinylestradiol (15 µg) was reported to lead to a decrease in sexual function in two studies, whereas higher doses (20-35 µg) were reported to increase sexual function in 13 studies, lead to no change in 4 studies, and cause a decrease in only one study.29 No differences were identified when comparing antiandrogenic with androgenic progestins,18,29 but associations with drospirenone, gestodene, and levonogestrel are sometimes reported to be associated with less symptoms of female sexual dysfunction.20,32
Most clinical trials included in this review report a positive or neutral impact of oral contraceptives in general,10,39 positive results for the association of ethinylestradiol with desogestrel,45 mixed results for the association of ethinylestradiol with drospirenone (better results for 20 µg than for 30 µg of ethinylestradiol),9,16,36 mixed results for the association of ethinylestradiol and levonorgestrel,15,45,49 and a negative result for the progestogen-only oral contraceptive with levonorgestrel.49 Regarding administration regimens, a shorter hormone-free interval seemed to be associated with better results.36
Non-oral hormonal contraceptives and female sexual function
Besides oral formulations, hormonal contraceptives are also available as transdermal patches, subcutaneous implants, intramuscular injections, vaginal rings, and intrauterine systems. Vaginal rings and transdermal patches exist only in combined estrogen and progestogen formulations, whereas the others are progestogen-only methods. Injections, implants and intrauterine devices are considered long-acting reversible contraceptive methods for their longer duration of action regardless of user action.26
Few studies exist regarding the effects of non-oral hormonal contraceptives on female sexual function, and evidence is still uneven.
The transdermal contraceptive patch exists as a combined hormonal method with an association of ethinylestradiol and norelgestromin, which is applied weekly for 3 weeks followed by a 7-day interval.26 The transdermal contraceptive patch has shown positive or neutral effects on female sexual function.18,19 The only recent clinical trial identified in this revision on the effect of the transdermal patch reported no association with lack of libido and, as such, no significant impact on female sexual function.10
The vaginal contraceptive ring exists as an association of ethinylestradiol and etonogestrel, which is applied intravaginally for 3 weeks, followed by a 7-day interval,26 and has previously shown mainly neutral effects on female sexual function.18,19,31 The clinical trials identified in this review regarding the effects of the vaginal contraceptive ring are summarized in Table 3. Most report a mainly positive impact of vaginal rings in female sexual function, improving desire, arousal, orgasm, satisfaction, lubrication, and pain.6,34,39 One study reported no significant variations in female sexual function,7 and one study reported negative results related to a decrease of interest in sex with the use of vaginal rings.10 Different dosages showed no difference, maybe because average hormonal release is the same (15 µg of ethinylestradiol and 120 µg of etonogestrel daily).34 An extended cycle with a 3-month interval-free administration followed by a 4-day interval resulted in better results.6
Table 3: Summarized analysis of the articles included in the review regarding vaginal contraceptive rings.
| Study | Method compared | Results | Conclusions |
|---|---|---|---|
| Guida et al, 201439 | Vaginal rings | Improvement in anxiousness, sexual pleasure, frequency and intensity of orgasm, satisfaction, sexual interest, complicity, sexual fantasy, number of sexual intercourses. | Positive impact |
| Caruso et al, 201934 | 3.47 mg ethinylestradiol/ 11.00 mg etonogestrel | Improvement of lubrication and orgasm at 3 months and of all FSFI parameters at 6 months. | Positive impact |
| 2.7 mg ethinylestradiol/ 11.7 mg etonogestrel | Improvement of desire, orgasm, satisfaction, and pain. | ||
| Caruso et al, 20146 | 2.7 mg ethinylestradiol/ 11.7 mg etonogestrel (extended 63-4-day cycle) | Improvement of desire, arousal, lubrication, orgasm, satisfaction, and dyspareunia; with an increase of FSFI and a decrease of FSDS. | Positive impact |
| Battaglia et al, 20147 | 2.7 mg ethinylestradiol/ 11.7 mg etonogestrel | No significant variation. | No impact |
| Boozalis et al, 201610 | Vaginal rings | Increased prevalence of lack of interest in sex. | Negative impact |
Notes: FSDS - Female Sexual Distress Scale, FSFI - Female Sexual Function Index.
Depot-medroxyprogesterone (DMPA) is a contraceptive method administered as an intramuscular injection every 12 weeks.26 Results on the impact on female sexual function are mixed, mostly neutral or negative.14,19,28,31 Two reviews reported no difference in sexual function, namely FSFI, desire, sexual interest, and arousal,14,31 while another reported loss of libido associated with the method, but no difference in dyspareunia.28 Two clinical trials were found for this review, both reporting a negative impact of DMPA injections on sexual function,10,49 affecting all FSFI domains except pain,49 as well as increased anxiousness before sex, a lack of interest in sex, and less pleasure in sex.10
The subcutaneous contraceptive implant exists as a progestogen-only contraceptive method with etonogestrel, which is applied subcutaneously in the arm for 3 years.26 Existing reviews report mostly negative sexual effects,19 with 2.5% of women having the implant removed due to decreased libido.14 The clinical trials included in this review regarding the effects of the subcutaneous contraceptive implant are summarized in Table 4, reporting mostly a positive or neutral effect on female sexual function,38-40 mainly with improvements in arousal, orgasm, satisfaction, and pain.38,39 One study reported negative results related to a decrease of interest in sex with the use of the subcutaneous implant.10
Table 4: Summarized analysis of the articles included in the review regarding subcutaneous contraceptive implant.
| Study | Results | Conclusions |
|---|---|---|
| Di Carlo et al, 201438 | Improvement of FSFI at 3 months, mostly in arousal, orgasm, satisfaction, and pain. | Positive impact |
| Guida et al, 201439 | Improvement of anxiousness, sexual pleasure, frequency and intensity of orgasm, satisfaction, sexual interest, number of sexual intercourses. | Positive impact |
| Higgins et al, 201640 | No difference in FSFI or NSSS. Subjective perception of no impact on sex life in 48% of women. | No impact |
| Boozalis et al, 201610 | Increased prevalence of lack of interest in sex. | Negative impact |
Notes: FSFI - Female Sexual Function Index, NSSS - New Sexual Satisfaction Scale.
The hormonal intrauterine systems exist with different doses of levonorgestrel - 13.5, 19.5, and 52 mg - and are placed inside the uterus for a period of 3, 5 or 5-7 years, respectively.26 All existing reviews report a positive effect of levonorgestrel intrauterine systems in female sexual function,14,18-20 with greater sexual desire14,18 and arousal.14 The clinical trials identified in this review regarding the effects of the hormonal intrauterine system are summarized in Table 5. These clinical trials are in accordance with the existing reviews, reporting a neutral or positive impact on female sexual function.10,37,40
Table 5: Summarized analysis of the articles included in the review regarding hormonal intrauterine system.
| Study | Method compared | Results | Conclusions |
|---|---|---|---|
| Caruso et al, 201837 | 13,5 mg levonorgestrel | Improvements in all aspects of sexuality and frequency of sexual activity at 6 months. Results were maintained at 12 months, except for dyspareunia that improved further. | Positive impact |
| Boozalis et al, 201610 | Hormonal intrauterine systems | No association with lack of libido. | No impact |
| Higgins et al, 201640 | Hormonal intrauterine systems | No difference in FSFI or NSSS. Subjective perception of no impact on sex life in 45%. | No impact |
Notes: FSFI - Female Sexual Function Index, NSSS - New Sexual Satisfaction Scale.
Non-hormonal contraceptives and female sexual function
Non-hormonal contraceptives include the copper intrauterine device and condoms. Other non-hormonal methods of contraception are the surgical sterilization procedures, tubal ligation in women and vasectomy in men.26 No studies or reviews were found regarding the impact of condoms. Regarding the copper intrauterine device, two studies report no impact on female sexual function, with no significant differences from baseline.40,49 Sterilization may have positive and negative psychological effects on sexual function.31 Vasectomy has led to an improvement in sexual arousal, satisfaction, orgasm, lubrication, and sexual desire,50 whereas tubal ligation led to lower FSFI scores, mainly in desire, lubrication, orgasm, satisfaction, and pain.43
Comparison between the effect of different contraceptive methods on female sexual function
One study comparing DMPA intramuscular injection, levonorgestrel intrauterine system, and copper intrauterine device found no major differences in sexual dysfunction at 3 and 12 months.51 Another study comparing the levonorgestrel intrauterine system and the copper intrauterine device also found a similar proportion of sexual dysfunction with both methods.47 One study comparing a combined oral contraceptive with 30 µg ethinylestradiol and 3 mg drospirenone with a 52 mg levonorgestrel intrauterine system showed better arousal, lubrication, orgasm, and satisfaction in the intrauterine device users, and lower FSFI score, desire, and interest, and higher pain in the oral contraceptive users.48 In these studies, no comparison with baseline values was performed, which did not allow to infer about improvement or worsening of sexual function with the introduction of the method.
Approach to women with sexual dysfunction attributed to contraception
Healthcare providers need to openly query women about sexual dysfunction, evaluate sexual function concerns, and consider alternative contraceptive options when needed.31 Predisposing, precipitating, and perpetuating factors should be explored,30 using a biopsychosocial model.19,30,31 The use of the FSFI is recommended.30 Physical examination and laboratory investigation should be performed when indicated.30
No guidelines exist for the management of sexual dysfunction associated with contraception. When contraceptive-related female sexual dysfunction is suspected, the recommended therapy is discontinuation of the contraceptive method,19,31,53 however this in isolation is not always feasible because it will leave the woman without a reliable contraceptive method to avoid pregnancy. As such, consideration of an alternative contraceptive method is peremptory. As with the initial prescription of any contraceptive method, the discussion of the advantages and side effects of specific methods is needed, and a shared decision should be made.
First, general lifestyle counseling may be useful, including setting aside time for connecting with the partner, increasing the woman’s exposure to sexual stimuli such as erotic literature or films, encouraging the maintenance of a healthy weight, ensuring adequate physical activity and sleep, enhancing skills for coping with stress, and recommending books women can use for self-education.19
When considering an alternative method of contraception, existing studies suggest that the hormonal intrauterine system, the copper intrauterine device, the implant, the vaginal ring, or permanent sterilization of either partner are all good options.19,20,31 Hormonal contraceptives with stronger androgenicity or shorter hormone free-intervals have shown to have a positive impact on sexual desire in women with decreased libido due to combined oral contraceptives.19,20,29,53
Since reduced androgen levels may play a role in contraceptive-associated sexual dysfunction, testosterone administration could restore baseline levels and, therefore, restore sexual function.8,46 Two studies evaluated the impact of adding dehydroepiandrosterone (DHEA) on female sexual dysfunction, but, despite a normalization of total and an increase of free testosterone levels, no statistical relevant differences from baseline sexual function were found.8,46 One of the studies reported an increase of genital sensations during erotic fantasy and less rejection of partner-initiated sexual encounters.46 From the existing evidence, the effects of DHEA administration appear to be more relevant in women with the highest physiological testosterone levels at baseline, as they will experience the strongest decreases.8
The role of estrogenic supplementation in contraceptive-related sexual dysfunction is not clear. In one study, the vaginal administration of a gel of estriol 50 µg/g resulted in an increase of the FSFI total score, as well as an improvement of dyspareunia.35 This improvement was also observed with the vaginal application of hyaluronic acid gel, although estriol has shown to be significantly more effective.35
In summary, existing evidence supports adequate counseling, replacement of the contraceptive method, and, in specific cases, pharmacologic treatment with DHEA or vaginal estrogens. In severe or refractory cases, consultation with a sex therapist may be helpful.31
Discussion
Contraception is widely used for birth control and is also used for the treatment of different gynecological disorders.7 Many studies evaluating the impact of contraceptive methods on female sexual function have been published throughout the times, but no clear conclusions have been found so far. The existing studies differ widely regarding population size and characteristics, the contraceptive method evaluated, and the parameters used for sexual function, which means that, when individual results are analyzed to draw specific conclusions, there is not enough quality data. As such, more quality and large-scale studies are needed, including all existing contraceptive methods, to allow for specific recommendations regarding this subject.
Considering the evaluated data, one can infer that contraceptive methods have an impact on female sexual function and that, depending on the contraceptive method and maybe even on the population, the effect may be positive, neutral, or negative. As previously mentioned, the estimated prevalence of contraceptive-induced female sexual dysfunction ranges from 5% to 20% worldwide,32 which means most women using contraceptive methods do not experience sexual dysfunction,10 but for the individual woman who is negatively affected, this can have a substantial impact on her quality of life and relationship.14
Regarding hormonal contraceptives, the analyzed studies report mainly positive or neutral effects on female sexual function regarding the levonorgestrel intrauterine system, the vaginal ring, and the transdermal patch. The subcutaneous implant and combined oral contraceptives led to mixed results, which studies reporting a good, neutral, or negative effect on female sexual function, and no specific associations were identified. The progestogen-only oral contraceptive and the DMPA intramuscular injections were associated mainly with negative sexual effects on women, even though not many studies were performed.
The impact of hormonal contraceptives on sexual function appears to depend on the quality and quantity of estrogen and progestogen, and on their regimen.36 The three groups of sex steroid hormones (estrogens, progestins, and androgens), are known to influence female sexual function via different effects on the vaginal tissue and the central nervous system.13 Ever since the introduction of hormonal contraceptive methods, the components have been modified to diminish the adverse metabolic effects on lipid and carbohydrate metabolism and hemostasis, and to reduce spotting and other menstrual disorders, but effects on sexual function have been different.9
Estrogen levels may have a direct or indirect impact on female sexual function. Labia minora, vaginal introitus, and clitoris are sensitive to estrogen modifications,9 as reduced estradiol levels in oral contraceptive users have been correlated with a decrease in clitoral volume and an increase in the dorsal clitoral artery pulsatility index, thus inferring a negative impact on sexual function.7 The estradiol plasma levels are not significantly decreased in non-oral hormonal formulations, such as the vaginal ring.7 This may in part justify the fact that the progressive ethinylestradiol reduction in the most recent formulations may have potential deleterious effects on female sexuality.7,9 Estrogens may also have an indirect impact through the increase of sex hormone binding globulin (SHBG) plasma levels and reduction of free androgen levels, producing hypoandrogenism.5 The reduction of circulating androgen levels is also caused by a direct reduction of androgen production from the ovary due to the action of systemic progestin.10,19,38 Androgens enhance sexual desire and response, but their impact depends on individual sensitivity,29 and the effect of their reduction, even though it is thought to be of decreased desire and arousability, is not as clear.7,8,14,16,29,46 A relative androgen deficiency can contribute to vulvar and vaginal dryness, decreased arousal, sexual pleasure, orgasm frequency, and increased sexual pain.31 However, in women with reduced levels of free testosterone, 20% showed a decrease in sexual desire and 80% had unchanged or increased sexual desire,18 which could mean that some women may be more sensitive to androgen level alterations than others.18,19 Hormonal contraceptives may also inhibit pituitary production and secretion of FSH and LH, reducing ovarian estradiol secretion and an absence of progesterone production,14 but this has not correlated with a change in sexual function.15 As such, the overall impact of an hormonal contraceptive on sexual function may reflect the balance between residual androgenicity of the progestin component and the magnitude of impact of the COC on SHBG, and, hence, free testosterone.53
Hormonal contraception can increase the risk of dyspareunia.27 Reduced concentrations of estrogens and androgens can lead to reduced activation of sex steroid receptors in peripheral tissues and vulvovaginal atrophy, leading to sensitivity to touch, burning, itching, and dryness during sexual activity,18 and an increased risk of vulvar vestibulitis, especially in those women with longer use, first use before 16 years of age, and when the pill used was of high progestogen, high androgen, and low estrogen content,27 as well as predispose to reduced vaginal lubrication.35 As an opposite, vaginal rings can lead to improved lubrication, which may be due to local activity of estrogens before entering the systemic blood flow and being metabolized.34 Contrary to this, there has also been a study showing a protective role of oral contraceptives regarding vulvodynia.42
Other ways that contraceptive methods may induce negative sexual changes may be related to the experience of increased vaginal bleeding.40 Also, some studies have been showing that women using hormonal contraceptives may differ in their mate preferences, reporting higher commitment to their current relationship, faithfulness or loyalty, and frequency of intercourse.30
The reported effects of non-hormonal contraceptives and even of vasectomy on female sexual function may suggest that other factors besides hormonal changes may be involved, including all domains of the biopsychosocial model mentioned above, as well as a possible regret that pregnancy is no longer possible when a definitive method is chosen.19
All these perspectives try to understand scientifically how contraceptives impact female sexual function, but so far, neither the effects nor the mechanisms behind them are completely understood. This review is in accordance with previously written reviews, which means that the additional evidence produced in more recent years is slowly giving support to previously made assumptions. Although, the need for more quality large-scale clinical trials remains, and hopefully in the future a new literature review performed will be able to create more definite conclusions, and clinical recommendations may be created regarding the role of each contraceptive method on female sexual function. Hopefully in the near future, more contraceptive methods will be developed, with even fewer negative side effects, allowing for the best physical, mental, and sexual well-being possible.
Conclusion
Even though the effects of the different contraceptive methods on female sexual function are not clearly established, it is safe to assume that, for some women, they may have a negative impact on their individual sexual function. A risk-benefit analysis of alternative contraceptive methods is important in those cases, and the identification of a contraceptive without adverse effects on sexual function should be preferred as first choice of treatment.15 Increased attention to sexual acceptability in contraceptive research could inform more personalized approaches to education, counseling, and decision support tools in the future.11
This review contributes to the increase of knowledge on this subject, mainly within family physicians, which will be more prepared to counsel their female patients with sexual concerns regarding contraceptive methods. First, professionals will be aware of the possibility of these negative effects on female sexuality. Second, knowing that this is a possibility, physicians will be mindful to openly ask patients about sexual concerns and sexual function, including pain, lubrication, or alterations in desire, following a biopsychosocial model of the possible factors predisposing to the symptoms. Regarding those patients who have not yet started a contraceptive method or will be undergoing a change of method, doctors will be alert that a baseline sexual history should be attained, to allow for posterior evaluation of possible side effects.