Introduction
To describe magnetic resonance imaging’s (MRI) role in the detection, characterization, and follow-up of liver metastatic disease in comparison to ultrasonography (US), computed tomography (CT), and positron emission tomography computed tomography (PET-CT).
Methods
A bibliographical review on the subject was performed, and abdominal studies from the author’s institutional PACS were selected as illustrative examples.
Results
The liver is the second-commonest site of metastatic disease dissemination, the first being the lymphatic system. Moreover, secondary lesions are found with greater frequency compared to primary liver tumors.1
The most common primary tumors that are associated with liver metastases are colorectal carcinoma (40%), stomach tumors (20%), pancreatic tumors (20%), breast tumors (10%), and lung tumors (10%).2
Imaging is essential in the detection and characterization of metastatic disease, determining the patient’s oncological stage. According to the most recent treatment options and contingent on multiple factors, patients with oligometastatic disease may undergo surgical metastatic resection or ablation with curative intent, making a precise determination of the number, location, and dimension of hepatic metastases crucial for treatment planning.3 Therefore, nowadays, the diagnosis of a metastatic liver is not enough, and a per-lesion detection is of paramount relevance.
Stage IV colorectal cancer (CRC) is characterized by the presence of distant metastases, which can be either confined to a single organ/location (stage IVa) or involve multiple organs/locations or the peritoneum (stage IVb). There has been a notable shift in the management of stage IV or metastatic CRC over the past decade, resulting in a substantial improvement in the overall survival of affected individuals. Specifically, the average survival duration has increased from less than 6 months to nearly 2 years.4 The success achieved in treating oligometastatic liver disease can be attributed, in part, to the enhanced utilization of hepatic surgery and/or local ablation, in addition to the implementation of new chemotherapy regimens. However, it is important to note that these interventions are only feasible following a comprehensive imaging evaluation.
Imaging is also determinant when it comes to evaluating chemotherapy and radiotherapy responses, as well as in the detection of recurrence after liver surgery or local ablation treatment.5
The presentation of hepatic metastatic disease encompasses a wide range of manifestations. Liver metastases often manifest as multiple focal lesions, although they can also occur as solitary masses or, less commonly, as confluent masses.2
Solid liver metastases can be classified as either hypovascular or hypervascular.1 Hypovascular metastases typically encompass CRC, gastric, and lung malignancies.
Hypervascular liver metastases can be found in renal cell carcinoma (particularly in clear-cell type tumors), neuroendocrine tumors, melanoma, thyroid carcinoma, and gastrointestinal stromal tumors. Breast cancer liver metastases may result in both hypovascular and hypervascular secondary hepatic lesions.
Cystic liver metastases often arise from cystic primary tumors, such as ovarian carcinoma and mucinous cystadenocarcinoma of gastrointestinal or pancreatic origin. These cystic secondary lesions may also arise from solid primaries such as gastrointestinal stromal tumor, leiomyosarcoma, malignant melanoma, carcinoid, and pheochromocytoma. Hepatic metastases with calcifications may result from ovarian or gastrointestinal mucinous adenocarcinomas and breast, lung, renal, and medullary thyroid carcinomas.4,6,7
This article aims to describe MRI’s role in the detection, characterization, and monitoring of liver metastatic disease in comparison to US, CT, and PET-CT.
Magnetic resonance imaging
MRI provides an excellent morphological and functional evaluation of the liver, making it extremely useful in the detection of hepatic metastases.
Diffusion-weighted imaging (DWI), a functional MRI sequence that informs on molecular activity and cellular function, can be useful in focal liver lesion detection and characterization, as well as in treatment response monitoring.8
Intravenous gadolinium-based contrast should be used in the setting of possible metastatic liver disease. Extracellular contrast agents are the most commonly used for most MRI studies, abdominal or not. These agents have shown a sensitivity of 90% and a specificity of 87% in the evaluation of hepatic metastases.9
Hepatospecific contrasts (Gadoxetate disodium/Primovist® and Gadobenate dimeglumine/Multihance®)
have shown even better results in metastatic liver lesion characterization. These gadolinium-based contrast agents enter normal hepatocytes, being able to determine whether focal hepatic lesions are hepatocellular or not, while also providing the same information extracellular contrasts allow. In a subsequent phase, they are partially excreted through the biliary tree, allowing for enhancement of hepatocytes, hepatocellular lesions, and biliary ducts when non-hepatocellular lesions show reduced enhancement.10
Gadoxetate disodium is rapidly excreted, leading to a fast excretory phase, allowing for a shorter exam duration. On the contrary, gadobenate dimeglumine has a long excretory phase requiring an acquisition at a later time, often needing two acquisition timings in the same day that may cause exam scheduling difficulties.
Despite that, gadobenate dimeglumine has a cost comparable to extracellular gadolinium contrast agents, while gadoxetate disodium has a significantly higher cost.11
Gadolinium-based contrast agents are related to fewer allergic adverse event occurrences in comparison to iodinated contrasts. Systemic nephrogenic fibrosis is an uncommon complication that is usually related to an underlying deficit in renal function. These occur mostly when utilizing class I gadolinium contrast agents. Class III agents are more recent and should be taken with some precautions, as there is not enough data regarding their possible adverse reactions as of yet. Gadoxetate disodium is a group III contrast agent and should be administered with a relative concern regarding the patient’s renal function. Class II agents such as gadobenate dimeglumine and most non-hepatospecific extracellular contrasts applied in clinical practice are safer.
Despite that, a glomerular filtration rate under 30 or a patient undergoing dialysis should warrant a cost-benefit analysis regarding the exam’s expected value.12
Abdominal MRI with hepatospecific contrast alongside DWI has shown a sensitivity of 98%, a specificity of 95%, and a negative predictive value of 100% in the detection of hepatic metastatic disease, being especially relevant in small hepatic lesions that may be missed in other modalities.13
PET-MRI is a relatively recent and noninvasive hybrid imaging method that superimposes PET’s radioisotope uptake with MRI’s excellent tissue characterization. It is still a markedly underutilized technique. Beiderwellen et al have found that it has sensitivity and specificity of 97% and 100% in the detection of hepatic metastases14,15 (Fig. 1).
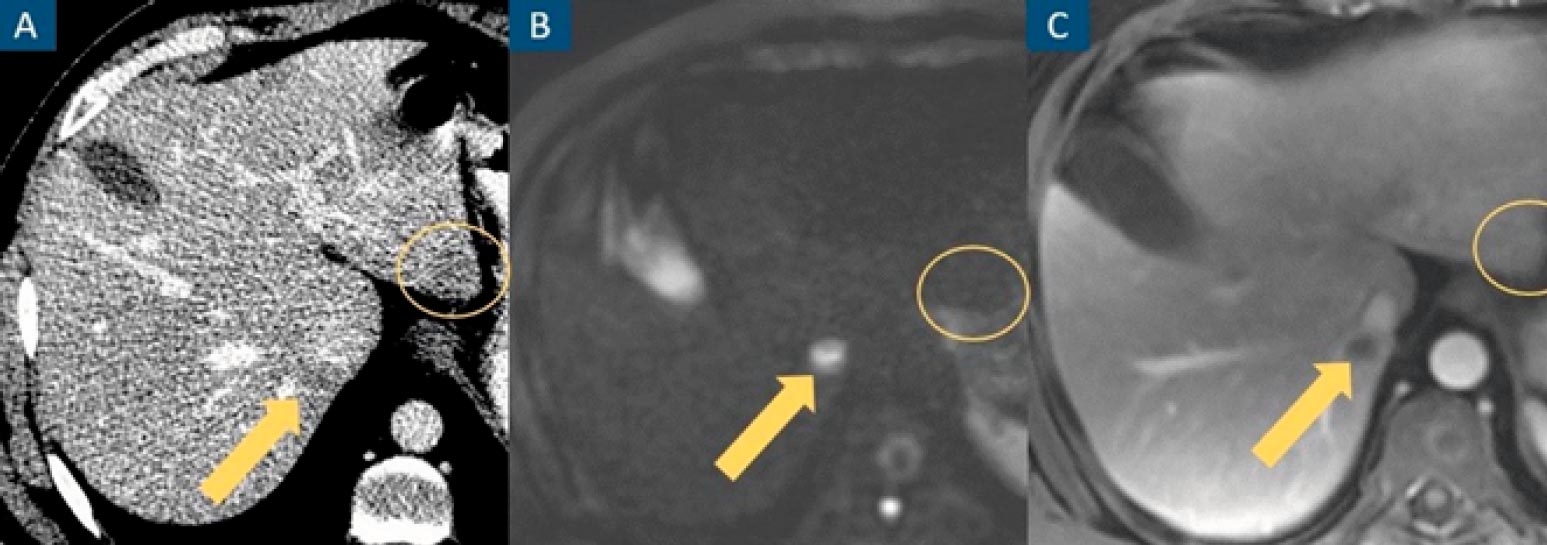
Figure 1: CT misses a true hepatic lesion and detects a pseudolesion. Axial contrast-enhanced venous phase CT image (A), axial diffusion-weighted image at b=1000 (B) and axial contrast-enhanced portal venous phase fat-suppressed T1-weighted image (C). A hepatic nodule (arrow) is inconspicuous on CT and evident on MRI. An apparent hepatic nodule on CT (circle) has no expression on MRI.
Ultrasonography
US is a widely available, low-cost, and safe imaging modality that may be used to evaluate liver metastases.
Despite these advantages, its dependence on operator and body habitus, poor detection of some lesions (namely isoechoic, small (<3-5 mm), and deep-seated lesions), inability to differentiate metastases from other primary liver tumors, and difficulty in precisely mapping lesions for future reference and comparison significantly limit its use for oncologic staging and follow-up.
Subdiaphragmatic lesions, chronic hepatic disease, and severe hepatic steatosis, often induced by chemotherapy, may also limit the characterization of metastatic disease. For these reasons, US has low sensitivity and specificity when it comes to detecting liver metastases (60%-65% and 50%-60%, respectively). Hepatic lesion characterization can be improved using contrast-enhanced ultrasound.2,16
Computed tomography
CT is the mainstay modality for liver metastatic disease evaluation, in part due to its fast acquisition, low cost, and widespread availability. Through contrast-enhanced multiphasic acquisitions, high-resolution images are obtained, which allow for the detection and mapping of hepatic lesions, liver volumetry, and evaluation of the rest of the body. Its disadvantages include the use of ionizing radiation, especially relevant in oncological patients that often require several follow-up studies, and the use of iodinated contrast, which can be limited in patients with renal insufficiency and has more adverse reactions in comparison to gadolinium-based contrasts. Some hepatic lesions cannot be accurately characterized, such as adenomas or focal nodular hyperplasias, requiring a further MRI evaluation. CT has a sensitivity of 79%-98% and a specificity of 77% in the detection of liver metastases from colorectal carcinoma.17
CT has shown limitations in the detection of liver metastases under 1 cm, especially after neoadjuvant chemotherapy and associated diffuse fatty infiltration of the liver18 (Fig. 2).
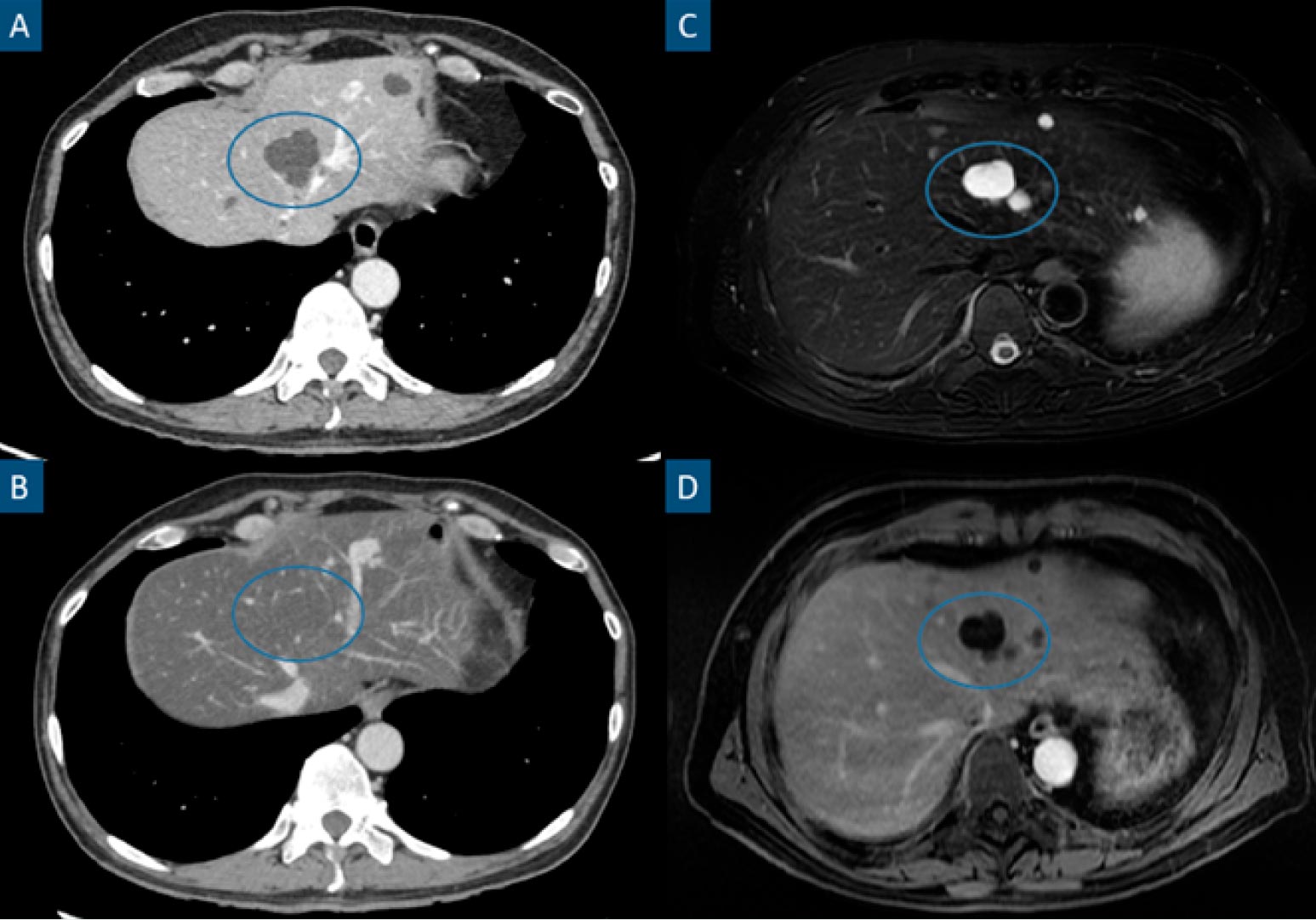
Figure 2: Steatotic liver hiding previously easily identifiable hypodense hepatic lesions in CT. Axial contrast-enhanced portal phase CT images (A and B), axial fat-suppressed T2-weighted image (C) and axial contrast-enhanced portalphase T1-weighted image (D). Patient with a stage IV clear cell renal cell carcinoma. A shows a hepatic cyst (circle), characterized by a hypodense, non-enhancing and well-marginated hepatic lesion. The patient underwent a chemotherapy cycle after the previously mentioned study and B was a follow-up CT performed 6 months later. B shows a noticeably steatotic liver, characterized by a low parenchymatous density. In this setting, hypovascular lesions such as cysts or most metastatic lesions may become inconspicuous as is the case in B. MRI (C and D) easily identify these lesions (circles) in the steatotic liver as T2 hyperintense non-enhancing lesions.
Positron emission tomography - computed tomography
PET-CT, namely using fluorine-18-labeled fluorodeoxyglucose (FDG), is less sensitive in detecting liver metastases (66%-93%) than CT and MRI, in part due to the poor detection of lesions under 1 cm and the poor detection of lesions after chemotherapy. This modality is useful in evaluating extra-hepatic disease. Other disadvantages include its elevated cost, low availability, and elevated ionizing radiation dose (higher than CT)19 (Fig. 3 and Table 1).

FIGURE 3: Liver metastasis undetected in intra-operatory hepatic ultrasound and PET-CT. Axial contrast-enhanced T1-weighted images on the arterial phase (A, D and G), and diffusion-weighted images at high b values (B, E and H) and ADC maps (C, F and I). Axial PET-CT with FDG (J). Patient with adenocarcinoma of the descending colon. Staging contrast-enhanced abdominal MRI was performed (A to F). A, B and C show a 20 mm highly restrictive hypervascular hepatic metastatic lesion in segment IVa (straight arrows). D, E and F show a 7 mm highly restrictive hepatic metastatic lesion in segment V (curved arrows). Intra-operatory hepatic ultrasound was performed only detecting the 20 mm lesion. Surgery consisting of a left hemicolectomy and single metastasectomy was performed. Histology revealed a metastatic adenocarcinoma lesion. Follow-up abdominal contrast-enhanced MRI performed 14 months later (G to I). G, H and I show an increase in size of the non-resected and previously described metastatic lesion in segment V (curved arrows). PET-FDG (I) acquired 3 days after the most recent MRI (G to I). No radioisotope uptake is detected in the previously mentioned lesion’s topography.
Table 1: Summary of the characteristics of each imaging modality.
Some studies have found that the more sensitive detection and better characterization of hepatic metastatic disease in colorectal cancer that MRI provides in comparison to triphasic CT allow for more accurate and cost-effective patient management. Saing et al developed a model with a defined population of patients with colorectal cancer and suspected liver metastases that demonstrated an increase in sensitivity and equal specificity in the evaluation of liver metastatic disease when comparing CE-MRI with CE-CT. These benefits translated into a significant cost-effectiveness gain in patient management, which was found despite the increased cost entailed by performing the exam. An even greater cost-effectiveness gain was described when utilizing gadobenate dimeglumine hepatospecific contrast.20
The VALUE Trial, conducted by Zech et al, and the study by Castell et al both reported comparable results. Zech et al compared extracellular contrast to gadobenate dimeglumine, while Castell et al compared it to gadoxetate disodium. These investigations specifically focused on patients diagnosed with colorectal cancer.21,22
More recently, similar results regarding the cost-benefit gains of contrast-enhanced MRI in comparison to contrast-enhanced CT were found in the hepatic metastatic evaluation of pancreatic cancer.23
It is the authors’ opinion that, provided the technique is available, oncological patient staging where hepatic metastatic disease is a concern should include an abdominal MRI, at least with extracellular contrast agents.
As above stated, an even more significant advantage in lesion characterization is achieved with hepatospecific contrast-enhanced MRI, however, its limitations may limit its use in some situations (Table 2).
Conclusion
In comparison to other imaging modalities, MRI is significantly more sensitive in the detection of hepatic metastatic disease, allowing for a more accurate and timely diagnosis even in the setting of small lesions (under 1 cm), hepatic steatosis, or post-chemotherapy patients, allowing for more cost-effective patient management. These advantages are even more evident when utilizing hepatospecific contrasts. Provided the technique is available, it is the author’s opinion that oncological patient management (staging or follow-up) where hepatic metastatic disease is a concern should include an abdominal MRI, if possible, utilizing hepatospecific contrast.24















