Introduction
Human beings have five basic senses: sight, hearing, smell, taste, and touch. Particularly, olfaction has three main functions: 1) to control food ingestion (e.g., determining food’s edibility, appetite regulation); 2) to avoid hazards (e.g., gas leaks); and 3) social communication (e.g., detecting body odors, sharing feelings).1 Although most patients with olfactory dysfunction (OD) are unaware of it, it is reported that around one-third of the patients with OD complain of reduced quality of life (QoL).2 These patients usually have worries regarding hazard avoidance, insecurities regarding their body smell and reduced enjoyment of food, which can consequently lead to anxiety and depressive symptoms. (3
OD is usually secondary to sinonasal disease, mainly chronic rhinosinusitis. However, other causes exist, such as congenital OD, posttraumatic OD, and post-infectious OD, which is trending since 2020 because of the COVID-19 pandemic. (4 Despite most COVID-19 patients having only transitory OD, some unlucky few are dealing with long-standing effects - a case-controlled study of 100 patients by Boscolo-Rizzo et al. reported that 7% had functional anosmia 1 year after infection. (5
The Questionnaire of Olfactory Disorders-Negative Statements (QOD-NS) with 17 items was an adaptation of the original version of the Questionnaire of Olfactory Disorders (QOD) by Frasnelli et al., which contained 52 items. (6,7 Recently, to improve efficiency of the questionnaire and reducing patient burden, Mattos et al. published the brief version of the QOD-NS (bvQOD-NS) with 7 items, with excellent correlations to the original scores. (8,9
To the best of our knowledge, there is no translation of the bvQOD-NS validated to the Portuguese language. Given the growth in awareness of the importance of OD and the increase in research, there is a need for standardized questionnaires to allow comparison between series and to better understand and deal with our patients’ struggles. (10 Thus, we aim to translate, validate, and adapt the bvQOD-NS to the Portuguese language.
Materials and methods
Study population
This study was approved by the Research and Ethics Committee of Centro Hospitalar do Tâmega e Sousa and followed the tenets of the Declaration of Helsinki for biomedical research. Every patient enrolled in the study agreed to participate in the study and signed the informed consent paper. Patients who were being followed in our ENT department between June and August of 2022 reporting OD were contacted to participate in the study. Exclusion criteria was refusal to participate and lack of cognitive skills necessary to complete the questionnaire.
Clinical and demographic variables were obtained from medical history with full ear, nose, and throat (ENT) examination, as well as through the patient’s records. All patients were submitted to psychophysical olfactory testing for odor threshold and identification, as recommended by Hummel et al. (4 For odor threshold we utilized the Connecticut Chemosensory Clinical Research Center (CCCRC) threshold test with butanol; (11 and for odor identification we used the Sniffin’ Sticks (SnSt) identification test with 16 pens (Burghart Messtechnik), which is already validated for the Portuguese population. (12,13
Translation and validation process of the Brief version of the Questionnaire of Olfactory Disorders-Negative Statements to Portuguese
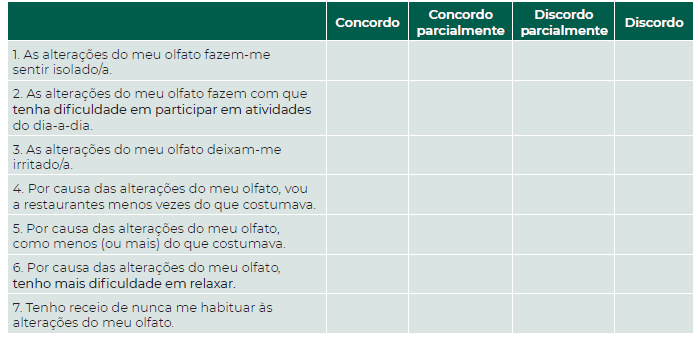
The score of the original bvQOD-NS is reported on a scale of 0 (I agree) to 3 (I disagree), with a maximum of 21 points. (8 Since the statements are negative, lower scores reflect worse olfactory-specific QoL. Recommendations for the translation and cross-culture adaptation of health-related QoL measures were followed to translate the original bvQOD-NS. (14
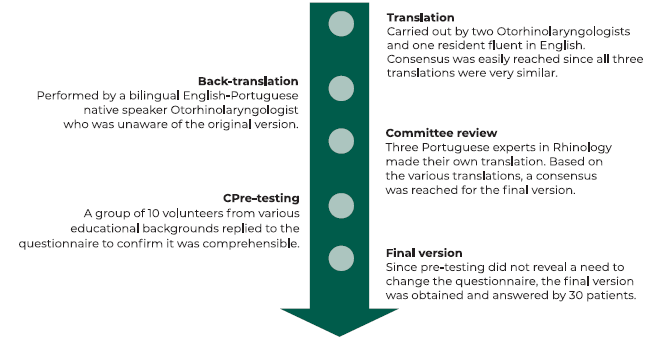
The translation and validation process is summarized in Figure 1. Independent translations were performed by three Portuguese doctors who are fluent in English. These three translations were reconciled into one preliminary Portuguese version, which was sent for back-translation by a bilingual English-Portuguese native speaker doctor. Since the original English version and the back-translated version were similar, the preliminary Portuguese version was then sent to three Portuguese experts in rhinology who are also fluent in English. Each made its own translation and compared the preliminary Portuguese version with the original one. After discussion between the translators, the rhinology experts and the back-translator, a final version of the Portuguese bvQOD-NS was obtained (Figure 2). Pre-testing was performed by asking ten volunteers with different educational degrees and no olfactory dysfunction to complete the questionnaire, and none of them reported any difficulty in the comprehension of the questions. The final version of the questionnaire was answered by thirty patients with OD in two different occasions: 1) at the time of olfactory testing; and 2) one month after completing the questionnaire, by teleconsultation.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 27.0. Armonk, NY. The Cronbach α coefficient was used to assess the internal consistency of the questionnaire, measuring the degree to which each question relates to the rest. The test-retest reliability, referring to the consistency between the scores of repeated measurements from the same participant, was assessed by the intraclass correlation coefficient. We used the independent Samples t Test to compare groups within the population.
Results
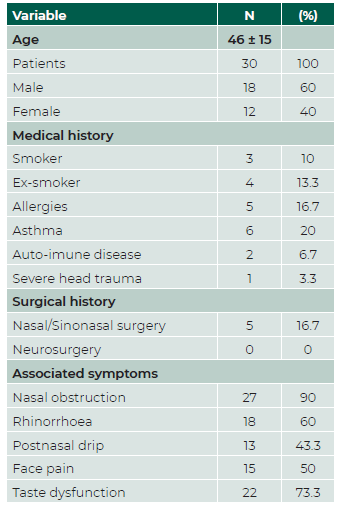
Thirty patients with olfactory dysfunction confirmed by psychophysical olfactory tests met the inclusion criteria. Mean age was 46 ± 15 and 18 (60%) were male. Patient demographic and clinical data are presented in Table 1. Every patient scored below normosmia in the odor threshold test with butanol, and the SnSt identification test revealed that our population was all below the 10th percentile according to the Portuguese normative data, indicating hyposmia/anosmia. (13 After ENT examination and imagiological evaluation with sinonasal computer tomography (CT) scan, patients’ etiologies of olfactory disorders were i) Chronic Rhinosinusitis (CRS) with nasal polyps (CRSwNP), ii) CRS without nasal polyps (CRSsNP), iii) post-COVID19 olfactory dysfunction, or iv) post-traumatic (Table 2). No patient was lost during the 1 month follow up.
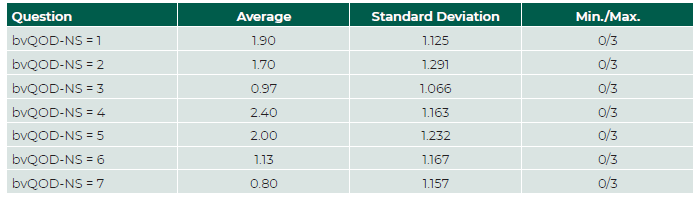
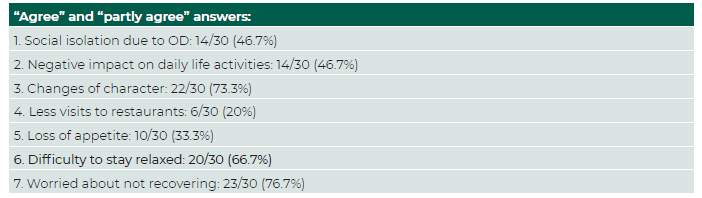
The Cronbach α of bvQOD-NS was 0.589 (<0.70), showing modest internal consistency of the questionnaire. The level of consistency was the lowest in questions four and five. The intraclass correlation coefficient was 0.925 (confidence interval [CI] 95%: 0.841-0.964), which is indicative of excellent test-retest reproducibility. (Table 3) Questions three and seven brought up more concern from the patients, whereas question four showed the least impact in their QoL. (Table 4 and 5).
Table 3 Internal consistency and intraclass correlation coefficient of the Portuguese version of the bvQOD-NS measured with Cronbach α.
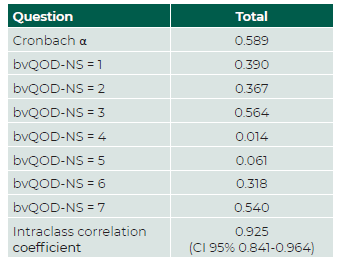
Table 5 Answers reflecting negative impact in QoL in each item of the Portuguese version of the bvQOD-NS.
Comparison of post-COVID19 patients with other causes of OD revealed a statistically significant difference (p<0.05) between the mean scores of the bvQOD-NS of both groups (8.1 vs 12.3).
Discussion
The greatest advantage of the bvQOD-NS in comparison with other olfactory specific QoL questionnaires is that is less time consuming, and thus it is likely to elicit better response rates and data quality. (15 While reviewing the literature, we could find that the bvQOD-NS was already translated to Spanish and French, highlighting its usefulness in clinical practice around Europe. (16,17 With an increasing interest in the study of OD and the lack of validated questionnaires for the Portuguese language, the seventh most-spoken first language in the world, the authors considered this questionnaire as the most adequate to be used in clinical practice and research. (18
Regarding the translation process, the authors tried to follow the guidelines of cross-cultural adaptation of QoL measures by Guillemin et al, (14 in order to come up with an equivalent translation of the original version to our country. However, there were some recommendations we chose not to implement: i) The authors did not feel it was necessary for the translation to be made by someone other than medical doctors, as all the translators were fluent in English and the back-translator was an English native speaker. The same process was made by Chiesa-Estomba et al. in the translation of the bvQOD-NS to Spanish; (16) ii) Similar to other QoL questionnaires that were translated and validated to Portuguese, translation process was done exclusively by medical doctors as we felt capable of doing it ourselves - and neither the pre-testing volunteers, nor the participants in the study reported any trouble in the comprehension of the questionnaire. (19,20
As a means to assess the reproducibility of the questionnaire, we decided that the participants should answer the Portuguese version of the bvQOD-NS in two separate occasions one month apart. The first time the questionnaire was completed by the patients was in the same moment they were submitted to olfactory testing. Since none of the participants reported any difficulty while answering the questionnaire, and because some patients were not willing to come in person to the hospital due to both time and money-consuming reasons, we decided that all the participants should complete the questionnaire for the second time by teleconsultation. Given the excellent test-retest reproducibility in our study, representing great temporal stability of the questionnaire, the authors believe the score of Portuguese version of the bvQOD-NS is not influenced by the way the questionnaire is answered, which can be extremely useful both in follow-up after treatment and in future scientific studies.
However, internal consistency was not as high as expected, particularly regarding eating questions (questions four and five), which were also the ones that showed least impact in QoL. We believe this might be linked with the low socioeconomic conditions of our population, as food enjoyment does not seem to be as related to hedonic-oriented motives (such as pleasure) as in higher socioeconomical groups. (21
Our study included patients with essentially two different etiologies of OD: CRS and post-infectious. Since SARS-CoV-2 infection was established as a possible cause of OD, the sense of smell that was previously overlooked started to be of major importance. (22,23 In our study there were 10 patients with post-COVID19 OD whom, in concordance with previous reports, revealed greater impact in QoL than the rest of the group. (15,24 This is thought to be due to the sudden onset of OD versus a more progressive dysfunction in patients with CRS. (15 Also, it is noteworthy that the patient who scored highest on the post-COVID19 group was the only one that reported getting psychological help specifically because of her recent OD. With previous studies linking post-COVID19 OD with isolation, stress and depression, psychotherapy might be useful in such cases and should be further investigated. (25
An important limitation of the present study is the small sample size, which probably influences the modest internal consistency we found in the Portuguese version of the bvQOD-NS. Another limitation is the follow-up period of only 1 month, which did not allow for an observation of changes in the olfactory specific QoL of our patients after treatment, either medical or surgical. Furthermore, our study did not include a control group. The reason why is that the questionnaire was created specifically for patients with OD, so the authors did not feel it was necessary to validate it, in concordance with other studies. (16 However, it would be interesting in the future to compare the results of a group of patients with OD to a control group in order to evaluate the discriminant validity of the questionnaire. Future studies with bigger sample sizes, including a longer follow up and evaluation pre- and post-treatment are needed to confirm the value of the Portuguese version of the bvQOD-NS.
Conclusion
Portuguese is the seventh most-spoken first language in the world. It is the authors belief that our translation into Portuguese of the bvQOD-NS will allow for a rapid and valid method to better understand the struggles and expectations surrounding olfactory dysfunction, ultimately benefiting the patients and providing important information for the medical community in future studies.