Introduction
Malignant tumors of the sinonasal tract are uncommon, comprising less than 1% of all malignancies and about 3% of head and neck malignancy 1,2.
These tumors can arise from a wide variety of tissues within the nose and paranasal sinuses, being defined as epithelial or nonepithelial in origin 3. Sinonasal malignancies occur predominantly in males, between 50 and 70 years of age 4.
Despite its rarity, in the last decades there has been significant advances in the diagnosis and treatment of sinonasal malignant tumors. However, survival remains poor 5. Nasal cavity and paranasal sinus tumors are commonly asymptomatic until they have extended beyond their bony confines, being diagnosed in later stages. In addition, the complex anatomy of the sinonasal region and proximity of critical structures such as the orbit, brain, or cranial nerves, further complicates management of these patients and leads to frequent local relapses and eventually death 6,7.
Comparison of results between different institutions is limited due to the rarity of these tumors, the presence of many different histologic subtypes and the advanced stage at the diagnosis 8.
The purpose of this study was to characterize the demography, risk factors, clinical presentation, histologic types, management, and survival of patients with malignant tumors of the nose and paranasal sinus treated at a tertiary hospital center in Portugal.
Materials and Methods
The study was conducted at Otolaryngology department of the Centro Hospital de Lisboa Ocidental, a Portuguese tertiary center.
The clinical records of patients with malignant tumors of the nose and paranasal sinuses were retrospectively reviewed through the Information Systems and Technologies Service (SSTI) of the Centro Hospitalar de Lisboa Ocidental. All patients with these malignancies, from January 2012 to December 2021, were included. The following data was collected: demographics (age, gender, occupation), habits (smoking, alcohol), clinical presentation, location, staging (according to the 8th edition of American Joint Committee on Cancer (AJCC) on epithelial tumors of the nose and paranasal sinuses and mucosal melanoma), histology (based on the 4th edition of World Health Organization (WHO) classification of tumors), treatment, residual disease, recurrent disease, five-year overall survival (OS) and disease-free survival (DFS) 9,10.
We excluded nasopharyngeal tumors because they have a different etiology, originating from epithelial and b-cell interactions of the nasopharynx, and the primary treatment in most cases is not surgical. We have also excluded basal cell carcinoma, squamous cell carcinoma (SCC) and melanoma of the nasal pyramid, because they are generally managed in the dermatology department 3.
Statistical analysis
Statistical analysis was performed with SPSS 24.0 for windows (SPSS Inc., Chicago, IL, USA). For overall survival (OS), the follow-up time was defined as the time between initial presentation at this institution for the tumor of interest and the last appointment or death. For disease-free survival (DFS), the follow-up time was defined as the time between the conclusion of treatment for the primary tumor until the date of the first recurrence, death, or last contact. Quantitative variables were expressed as mean ± standard deviations or as median ± interquartile range for data not normally distributed. Qualitative variables were expressed as absolute values and percentages. Kolmogorov-Smirnov and Shapiro Wilk normative tests were used in order to access distribution pattern in quantitative variables. Student’s t test, A-nova one way and respective non-parametric tests, χ2 and proper adjustments were used to relate evaluated variables with primary outcomes (death and recurrence). Kaplan - Meyer curves were used to determine 5-year OS and DFS and qualitative variables were compared regarding survival. A p value < 0.05 was considered statistically significant.
Results
From January 2012 to December 2021, 19 patients were included in our study (table 1). The patients were predominantly males (11 patients, 57.9%), with a mean age at diagnosis of 66.7 ± 9.8 years (range between 51 and 89 years). Ten patients (52.6%) were smokers and 6 (31.6%) had alcoholic habits. Four patients (21.1%) had occupational risk factors (2 wood workers, 1 working in textile industry and 1 working with chemical compounds) and 2 patients (10.5%) had history of previous tumors.
The most common presenting symptoms were nasal obstruction and epistaxis (both present in 9 patients, 47.4%). The overall variety of presenting symptoms is summarized in table 2.
Table 1: Characteristics of the patients included
| Characteristics | n=19 |
|---|---|
| Age (years) | 66.7 (range 51-89) |
| Male (number and %) | 11 (57.9%) |
| Smokers (number and %) | 10 (52.6%) |
| Alcoholic habits (number and %) | 6 (31.6%) |
| Occupational risk factors (number and %) | 4 (21.1%) |
| History of previous tumors (number and %) | 2 (10.5%) |
Table 2: Presenting symptoms
| Clinical manifestation | Number of patients (%) |
|---|---|
| Nasal obstruction | 9 (47.4%) |
| Epistaxis | 9 (47.4%) |
| Facial swelling | 3 (15.8%) |
| Headache | 3 (15.8%) |
| Rhinorrhea | 2 (10.5%) |
| Proptosis | 2 (10.5%) |
| Hyposmia | 1 (5.3%) |
| Incidental finding | 1 (5.3%) |
In all patients (19, 100%), the sinonasal malignancy represented a primary tumour. The most common location was the nasal cavity (9 patients, 47.4%), followed by maxillary sinus (4 patients, 21.1%) (table 3).
Staging for epithelial tumors of the nose and paranasal sinuses and mucosal melanoma is summarized in table 4. Regarding the T component of the TNM staging classification, T3 was the most common at presentation (5 patients, 35.7%), followed by T4b (4 patients, 28.6%). There were 2 patients (14.3%) with nodal disease at presentation and no patient presented with distant metastasis.
Histologic classification of the tumours according to the World Health Organization is depicted in table 5. Epithelial malignancies were the most common (11 patients, 57.9%), followed by neuroectodermal malignancies (5 patients, 26.3%) and soft tissue malignancies (3 patients, 15.8%). Squamous cell carcinoma (SCC) was the most common histologic subtype (4 patients, 21.1%), followed by adenocarcinoma, adenoid cystic carcinoma and mucosal melanoma (each present in 3 patients, 15.8%). Transformation of inverted papilloma into squamous cell carcinoma occurred in 3 of 4 patients. Of these patients, one was referred to our hospital with a history of 2 previous surgeries 30 years ago for nasal polyposis and inverted papilloma diagnosed histologically and subsequent recurrence of a sinonasal mass, the other was referred after biopsy of a sinonasal mass compatible with inverted papilloma and subsequent partial excision of the mass with pathological analysis showing an inverted papilloma with areas of transformation into a squamous cell carcinoma and the last was referred after an incidental diagnosis in a CT scan of the head revealing an expansive mass in the left maxillary sinus with bone erosion and subsequent invasion of pterygopalatine fossa and osteoneogenis, in probable relation with inverted papilloma.
Table 3: Tumor location
| Site of origin | Number of patients (%) |
|---|---|
| Nasal cavity | 9 (47.4%) |
| Maxillary sinus | 4 (21.1%) |
| Overlapping | 3 (15.8%) |
| Ethmoid sinus | 1 (5.3%) |
| Frontal sinus | 1 (5.3%) |
| Sphenoid sinus | 1 (5.3%) |
Table 4: TNM staging at presentation for epithelial tumors and mucosal melanoma according to the 8th edition of American Joint Committee on Cancer (AJCC). For mucosal melanoma, all tumors are classified at least as T3.
| TNM staging | Number of patients (%) |
|---|---|
| T1 | 3 (21.4%) |
| T3 | 5 (35.7%) |
| T4a | 2 (14.3%) |
| T4b | 4 (28.6%) |
| N+ | 2 (14.3%) |
Table 5: Histologic classification according to the 4th edition of World Health Organization (WHO) classification of tumors
| Site of origin | Number of patients (%) |
|---|---|
| Epithelial malignancies | 11 (57.9%) |
| Squamous cell carcinoma | 4 (21.1%) |
| Adenocarcinoma | 3 (15.8%) |
| Adenoid cystic carcinoma | 3 (15.8%) |
| Neuroendocrine tumors | 1 (5.3%) |
| Soft tissue malignancies | 3 (15.8%) |
| Angiosarcoma | 1 (5.3%) |
| Leiomyosarcoma | 1 (5.3%) |
| Rhabdomyosarcoma | 1 (5.3%) |
| Neuroectodermal malignancies | 5 (26.3%) |
| Mucosal malignant melanoma | 3 (15.8%) |
| Olfactory neuroblastoma | 2 (10.5%) |
Surgical treatment was the preferred primary modality of treatment (16 patients, 84.2%). Nine patients (56.3%) were submitted to external approach, while 7 patients (43.8%) underwent endonasal endoscopic treatment. In table 6 is summarized the histology and local staging for patients that underwent surgical treatment as the initial treatment. Pathologic analysis revealed microscopic positive margins in 8 patients (50%), negative margins in 6 patients (37.5%) and not evaluable in 2 patients (12.5). Neck dissection was performed in 2 patients (12.5%) with N1 disease, one with squamous cell carcinoma and the other with mucosal malignant melanoma. In patients submitted to primary surgical treatment, adjuvant radiotherapy was performed in 10 patients, whereas adjuvant chemoradiotherapy in 2 patients.
Non-surgical treatment was the treatment of choice in 3 patients (15.8%). In this subgroup, 1 patient received radiotherapy, 1 chemoradiotherapy and 1 chemotherapy (table 7).
Table 6: Histology and local staging for patients submitted to surgery as initial treatment
| Histology | Local staging (TN) |
|---|---|
| External approach (n=9) | |
| Leiomyosarcoma (n=1) | Not applicable |
| Squamous cell carcinoma (n=2) | T1N0; T4aN1 |
| Adenocarcinoma (n=1) | T3N0 |
| Neuroendocrine tumors (n=1) | T4bN0 |
| Adenoid cystic carcinoma (n=2) | T3N0; T4bN0 |
| Angiosarcoma (n=1) | Not applicable |
| Olfactory neuroblastoma (n=1) | Not applicable |
| Endonasal approach (n=7) | |
| Squamous cell carcinoma (n=1) | T4aN0 |
| Adenocarcinoma (n=2) | T3N0; T1N0 |
| Adenoid cystic carcinoma (n=1) | T1N0 |
| Olfactory neuroblastoma (n=1) | Not applicable |
| Mucosal malignant melanoma (n=2) | T3N0; T3N1 |
Table 7: Primary treatment modalities
| Treatment | Number of patients (%) |
|---|---|
| Surgical treatment | 16 (84.2%) |
| Surgery alone | 4 (21.1%) |
| Surgery + radiotherapy | 10 (52.6%) |
| Surgery + chemoradiotherapy | 2 (10.5%) |
| Non-surgical treatment | 3 (15.8%) |
| Radiotherapy | 1 (5.3%) |
| Chemoradiotherapy | 1 (5.3%) |
| Chemotherapy | 1 (5.3%) |
Mean follow-up period of patients was 26.9 ± 20.6 months (range from 2-71 months). Recurrent disease occurred in 5 patients (26.3%), being local in 3 patients and metastatic in 2 patients. In table 8 is summarized the histology, initial TNM staging and the local of recurrence.
Table 8: Histology, initial TNM staging and local of recurrence for patients with recurrent disease
| Histology | Staging (TNM) | Recurrence |
|---|---|---|
| Squamous cell carcinoma | T1N0M0 | Local (premaxillary skin, maxillary bone, hard palate, nasal cavity floor) |
| Neuroendocrine tumors | T4bN0M0 | Distance (epicranium) |
| Mucosal malignant melanoma | T3N0M0 | Distance (lung) |
| Mucosal malignant melanoma | T3N1M0 | Local (hard palate) |
| Adenoid cystic carcinoma | T4bN0M0 | Distance (lungs) |
Five-year overall survival (OS) was 53.5% and disease-free survival (DFS) was 62.8%. History of previous tumors was statistically associated with worse prognosis (p < 0.001), while smoke (p = 0.733), alcohol consumption (p = 0.205) or occupational risk factors (p = 0.584) did not affect prognosis. Patients with nonepithelial tumors had worse prognosis compared with patients with epithelial tumors, despite the difference was not statistically significant (p = 0.230). N component of TNM staging for epithelial and mucosal melanoma tumors significantly affected 5-year OS, with positive nodes associated with worse prognosis (p = 0.025, figure 1a), as well as DFS (p = 0.025, figure 1b). While patients with T3, T4a or T4b tumors had worse prognosis compared with T1 patients, the difference was not statistically significant (p = 0.092, figure 1c). Primary treatment modality significantly influenced survival (p = 0.002), with surgical therapy showing a statistically significant difference compared to nonsurgical treatments (p < 0.001). In patients submitted to surgical treatment, there was no statistically significant difference in 5-year OS between external approach and endonasal endoscopic approach (p = 0.724). Although the presence of microscopically positive margins was associated with worse prognosis, the difference was not statistically significant (p = 0.123).
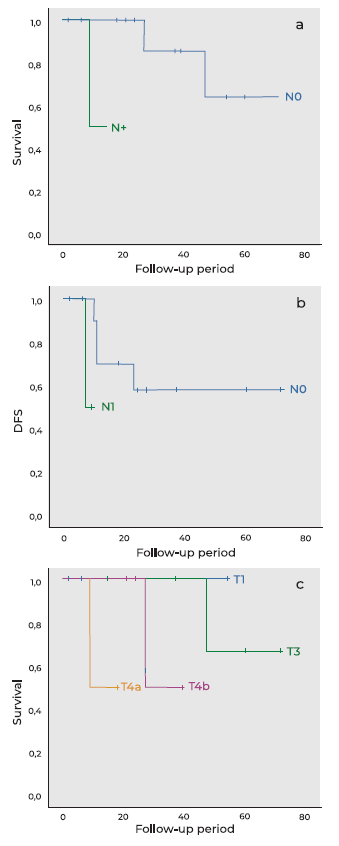
Figure 1 Kaplan-Meyer curves for different component of TNM staging for epithelial and mucosal melanoma tumors. a: five-year overall survival curve for N component (p = 0.025); b: Diseasefree survival course for N component (p = 0.025); c: five-year overall survival curve for T component (p = 0.092).
Discussion
There are few published reviews of malignant tumors of the nose and paranasal sinuses, mainly due to the rarity of these tumors and highly histologic diversity 4. According to the literature, the peak incidence of these tumors occur in the 5th to 7th decades, which is according to our results 11. There is evidence that occupational risk factors contribute to carcinogenesis of sinonasal malignant tumors. While adenocarcinomas have been linked to wood dust, formaldehyde and leather dust, squamous cell carcinomas have been associated to arsenic and welding fumes 12,13. Our series showed a slightly male predominance (57.9%), which is in conformity with literature. While in other tumors of the head and neck region, such as laryngeal, oropharyngeal or hypopharyngeal carcinomas it is reported a male predominance of over 90%, in malignant tumors of the nose and paranasal sinuses the male predominance is lower, probably because in this latter region, tobacco and alcohol (classically more associated to males) do not have a high carcinogenic potential 8,14.
The retrospective analysis of 13.295 patients performed by Dutta et al revealed that the most common origin of sinonasal malignancies was the nasal cavity (45.7%) followed by the maxillary sinus 7. However, the most common location of these malignancies is controversial, since other studies reported the maxillary sinus as the most common site 15,16. These studies also report the ethmoid sinus as a common location for this malignancy. Our results demonstrated a predominance for the nasal cavity (47.4%), followed by the maxillary sinus (21.1%). The ethmoid sinus was an uncommon location (1 patient, 5.3%), probably because tumors that originate in this region easily gain access to nasal cavity and subsequently were classified as overlapping (15.8%).
Most series report nasal obstruction as the most common presenting symptom 17,18. In our series, both nasal obstruction and epistaxis were the most common initial symptoms.
Histology of sinonasal malignancies was classified according to the 4th edition of the WHO 10. In our study, epithelial tumors were the most common histologic type (11 patients, 57.9%), whereas squamous cell carcinomas (SCC) were the most common histologic subtypes (4 patients, 21.1%). The predominance of epithelial tumors and of squamous cell carcinomas is in line with the most published series in the literature 4,7,8,16,19.
We used the 8th edition of American Joint Committee on Cancer (AJCC) on epithelial tumors of the nose and paranasal sinuses and mucosal melanoma to classify the staging of epithelial tumors and mucosal melanoma. The latter is very aggressive and carries a poor prognosis, which makes that all tumors are classified at least as a T3 and stage III 9. Patients with sinonasal malignancies usually present with advanced disease, because of its silent pattern of growth. In the earlier stages of the disease, when there are signs and symptoms, they are usually nonspecific, similar to benign sinus disease 2,4,6. Therefore, it is necessary a high clinical suspicion to make an early diagnosis 20. In most series, epithelial tumors of the sinonasal tract are diagnosed when they are locally advanced, which means a T3 or T4 lesion 15,16. This is in accordance with our results, since 11 patients (78.6%) were diagnosed with a T3 or T4 lesion, while 3 patients (21.4%) were diagnosed with a T1 lesion. All T1 lesions were located in the nasal cavity. This probably occurred because as lesions in the nasal cavity grow, they can produce symptoms such as nasal obstruction earlier, comparatively to tumors that grow in paranasal sinuses. In several studies, T component of TNM staging system was found to be a significant predictor of prognosis 4,15. Although we found a tendency for worse prognosis in T3, T4a or T4b lesions compared to T1 lesions, the difference was not statistically significant (p = 0.092). We suspect that this happened because of the small number of patients with early-stage disease. On the other hand, nodal stage (N component of TNM) was also reported to be a significant factor of prognosis, which is in conformity with our results (p = 0.025) 15,16.
Treatment of sinonasal malignancies is made on a case-by-case basis, taking into account several factors, such as histology, staging, feasibility of complete surgical resection and treatment risks and morbidity 6. Similarly to other reports, most patients underwent surgical treatment (16 patients, 84.2%), being the surgical therapy followed by adjuvant radiotherapy the most common modality (10 patients, 52.6%) 17,18. Our results are in agreement with other series, which showed a better prognosis for surgical therapy compared to nonsurgical therapy (p < 0.001) 15,18. Historically, the gold-standard surgical procedure for sinonasal malignant tumor has been the open craniofacial approach. Over the past decade, there has been increasing evidence regarding the effectiveness and safety of endoscopic endonasal approaches. Several studies demonstrated survival rates comparable to those of open surgery in carefully selected patients, with reduced morbidity and increased quality of life 5,21,22. Comparative studies between endoscopic and external approaches are limited by multiple factors, including location, surgeon experience and TNM degree; considering these variables, is still relevant to report that our study found no statistically significant difference in 5-year OS between open approach and endoscopic approach (p = 0.724). According to Paolo Castelnuovo et al., endoscopic endonasal approach allows resection of T1-T3 lesions, as well as selected T4a, being contraindicated as exclusive approach in cases where there is infiltration of nasal bones and palate, extensive involvement of the frontal sinus or the lacrimal pathway, extension into the infratemporal fossa and involvement of orbital content 23.
In concordance with other series, the treatment failure was mainly due to local recurrence. Our study reports a 5-year OS of 53.5%, with results from literature varying from 38-60% 4,15,16,17,18.
The main limitations of this review are its retrospective nature and the relatively small sample size, which can be easily explained by the fact that sinonasal malignancies are rare entities. These facts, allied to the wide histopathological diversity, the complex anatomy of the region, and the different surgical techniques available, difficult comparison of results.
Conclusion
Despite the recent advances in staging, histological classification, imaging modalities for diagnosis and surgical techniques, the prognosis of these rare malignancies remains poor. The present study has shown that prognosis is stage and treatment-dependent.
Conflito de Interesses
Os autores declaram que não têm qualquer conflito de interesse relativo a este artigo.
Confidencialidade dos dados
Os autores declaram que seguiram os protocolos do seu trabalho na publicação dos dados de pacientes.
Proteção de pessoas e animais
Os autores declaram que os procedimentos seguidos estão de acordo com os regulamentos estabelecidos pelos diretores da Comissão para Investigação Clínica e Ética e de acordo com a Declaração de Helsínquia da Associação Médica Mundial.















