Introduction
SARS-CoV-2 emergence raised a significant public health challenge that defied society´s limits as one knew it 1. Studies have shown that SARS-CoV-2 can directly infect and damage olfactory sensory neurons and the supporting cells and blood vessels in the olfactory epithelium 2. Indeed, COVID-19 has been associated with olfactory complaints, including anosmia, hyposmia and dysosmia. These symptoms have been reported in up to 85% of COVID-19 patients and are often some of the earliest and most specific indicators of infection 3. Although initially assumed as transient by many authors 4,5 it is now known that about 15% of infected patients do not recover for several months 5,6. Then, many COVID-19 patients may experience long-term changes in their smell and taste perception, which adds to the ongoing burden of long COVID-19 (7). Certainly, this underlooked “olfactory pandemic” persists nowadays and calls to be combated. With the former in mind, unravelling treatment solutions for long-lasting olfactory impairment has never seemed more urgent 1. Various therapeutic modalities have been suggested to treat persistent COVID-19 induced olfactory dysfunction (pCIOD) (1,8. These include topical nasal corticosteroids, intranasal vitamin A + E , vitamin B complex tablets, among others 1. Nevertheless, besides olfactory training, no adjuvant therapy has unequivocally shown benefit 1,8.
Recently, a novel approach for treating persistent olfactory dysfunction has been described : injection of platelet rich plasma (PRP) into the olfactory mucosa 9,10. PRP is an autologous blood product containing supraphysiologic levels of growth factors and cytokines 11,12. It has been shown to promote tissue regeneration and repair and has been medically used in various clinical scenarios , such as in osteoarticular pathology, skin rejuvenation, wound healing, and hair regrowth 11,12. PRP injection was proposed as a potential new therapy for olfactory dysfunction treatment in 2019, when Yan et al brought the first pilot study with preliminary promising results 9. The same group published the first randomized trial on PRP application in late 2022, with favorable results 10
The main objective of this work is to describe the resulting olfactory outcomes of pCIOD patients submitted to PRP injection while addressing any possible ensuing complications. A distinctive, customized protocol was developed at the smell and taste consultation of Centro Hospitalar Universitário de Santo António, and its implementation is outlined as follows.
Material and Methods
Sample enrollment and evaluation
Patients who attended the Otorhinolaryngology Department's smell and taste clinic at Centro Hospitalar Universitário de Santo António were evaluated for eligibility. Data related to anamnesis and physical examination was collected according to the institutional protocol described elsewhere 1.
The inclusion criteria were: age ≥18 years, abrupt onset of olfactory impairment coincident with SARSCoV-2 infection verified by nasal swab and Polymerase Chain Reaction technique (PCR), subjective persistence of pCIOD ≥ 12 months, objective olfactory perception threshold (OPT) ≤ 7 at the time of PRP injection, having performed olfactory training and adjuvant therapy without achieving substantial improvement (measured by at least two consecutive 3 months apart OPT evaluations) and cognitive ability to sign informed consent. Exclusion criteria were as follows: Chronic rhinosinusitis, history of head trauma with loss of consciousness, documented pre-existing olfactory dysfunction before COVID-19, pregnancy, previous neurosurgery or endoscopic nasal surgery, known olfactory bulb lesions on imaging, known neurologic disease (Parkinson's, Dementia, Epilepsy), major psychiatric disease, inability to tolerate nasal endoscopy, known platelet or coagulation disorders, undergoing anti-inflammatory or anticoagulant therapy, and concurrent pathology presumably needing anti-inflammatory treatment in the next 30 days following injection.
A visual analog scale (VAS) was used to subjectively assess olfactory impairment on a scale from 0 to 10, with participants asked to rate their satisfaction with their smell and taste function (10 indicating a return to normal). This measurement was taken at three time points: immediately before the injection, one month after, and three months after the injection. The olfactory perception threshold (OPT) was measured in the same time points by means of Burghart Sniffin' Sticks n-butanol threshold test with 16 levels (48 pens), a validated method. Furthermore, nasal endoscopy was performed at each time point to rule out baseline structural pathology such as polyposis, which would exclude the patient from the cohort, and evaluate possible local complications of the procedure.
PRP preparation and injection procedures
To obtain a reproducible procedure for clinical practice application, the authors performed a primary comprehensive literature search. Additionally, an experienced physician in osteoarticular PRP injections was present throughout the first three cases to ensure adequate PRP sample preparation. Patients were seated in an upright position as shown in Figure 1. The technique started with venous blood extraction onto three different 3.5 ml tubes containing 3.2% sodium citrate. PRP samples were obtained through a 10-minutes continuous centrifugation at 4000 rotations per minute. The PRP samples were then drawn into two separate 1‐mL syringes until the 0.9 ml mark was reached and a 27‐g needle was adapted for injection (Figure 2).
A 30º rigid nasal optic was used to inspect the patient’s nasal cavity to visualize and predict the injection site. Both the inferior meatus and predicted injection site were anesthetized using lidocaine + prilocaine and phenylephrine hydrochloride eluted cotton pledgets, carefully placed by endoscopic visualization and maintained in situ for ≥ 10 minutes. Under endoscopic control, a single-site injection was performed along the superior nasal septum posterior to the head of the middle turbinate, as shown in Figure 3. The needle was aimed at the nasal septum at the level of the superior two-thirds of the middle turbinate (see Figure 3). All the procedures were done by the same investigator holding the endoscope and the needle, while an assistant pulled the embolus after puncture and aspiration to deliver 0.9 ml of PRP on each side of the nasal septum. The patient was asked to rate the overall procedure discomfort on a scale of 0 to 10 immediately after the second injection. Following the procedure, patients were observed for 15 minutes to monitor for any potential adverse effects before being discharged.
Ethics
Informed consent was obtained for all patients. The local Ethics Committee approved the study (Number: 2021.93(075-DEFI/078-CE)) and the design complies with the Declaration of Helsinki´s ethical standards.
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics 29). In the descriptive analysis, categorical variables are presented as percentages, and continuous variables as means and standard deviations, since normal distribution was confirmed by using skewness and kurtosis. A bivariate analysis regarding baseline sample characteristics versus olfactory outcomes measured by OPT and VAS scores was undertaken. The associations were analyzed using either independent t-test (parametric analysis), Pearson Chi-square/ Fisher´s tests (95% confidence intervals) for categories and Spearman´s test for continuous variables. Finally, a repeated measures ANOVA was performed in order to bring a general linear model based on OPT and VAS values across the three follow-up timepoints. All reported p values are two-tailed, with a p value ≤ 0.05 indicating statistical significance.

Figure 2 Platelet rich plasma (PRP) prefilled syringes after centrifugation (one for each nasal cavity).
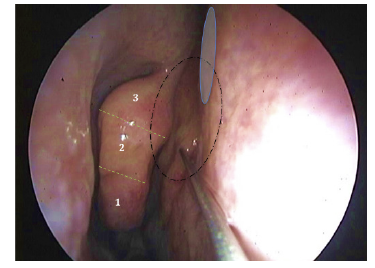
Figure 3 Single-site injection being performed in the right superior nasal septum, posteriorly to the level of the head of the middle turbinate. PRP dispersion can be perceived by the resulting mucosal blanching in a superior and posterior direction (dashed circular line). Care must be taken to avoid pinching the presumable site of S-point (blue circle), which can occur if the puncture occurs too highly in the septal mucosa. Dashed green lines and numeration are displaying three virtually marked vertical divisions of the middle turbinate - the injection is performed at the level of the superior part of the middle portion (labeled as 2).
Results
A total of 20 patients were enrolled. A general description of the main variables is displayed on Table 1. The mean age at injection was 44.64 ± 9.52 years. Mean OPT before injection was 4.36 ± 1.6. There was a statistically significant difference between baseline OPT and the 1-month OPT in the paired-samples t-test (mean incremental change: 2.67 ± 2.35, p =0.012). Likewise, there was a statistically significant difference between baseline OPT and the 3-months OPT in the paired-samples t-test (mean incremental change: 3.98 ± 1.92, p = 0.009). The mean VAS baseline score was 4.1 ± 1.8. There was a statistically significant difference between baseline and the 1-month VAS scores in the paired-samples t-test (mean incremental change: 2 ± 2.18, p =0.025). Likewise, there was a statistically significant difference between the baseline and the 3-months VAS scores in the paired-samples t-test (mean incremental change: 3.5 ± 2.54, p = 0.005). A positive association was found between baseline OPT and baseline VAS score (p = 0.021). However, no association was found between 1-month OPT and 1-month VAS score (p = 0.8). The 3-months OPT and VAS score showed a tendency for correlation, although not reaching statistical significance (p = 0.057). All patients reported only mild or no discomfort related to the injection (VAS score for procedural pain ≤ 2).
No association was found between baseline to 1-month OPT variance and sex (p = 0.181), age (p =0.342), time from COVID-19 diagnosis to PRP injection (p = 0.763), or any comorbidity (p >0.05). Likewise, no significant correlation existed between baseline to 3-months OPT variance and sex (p=0.357), age (p=0.864), time from COVID-19 diagnosis to PRP injection (p = 0.694) or any comorbidity (p>0.05). No association was found between baseline to 1-month VAS score variance and sex (p = 0.378), age (p =0.876), time from COVID-19 diagnosis to PRP injection (p = 0.982), or any comorbidity (p >0.05). Likewise, no significant correlation existed between baseline to 3-month VAS variance and sex (p=0.472), age (p=0.753), time from COVID-19 diagnosis to PRP injection (p = 0.881) or any comorbidity (p>0.05).
A general linear model was furtherly created to predict the OPT and VAS scores through the three measured time points (Figure 4). A repeated-measures ANOVA determined that mean OPT values differed significantly across the three time points (F (2,8) = 10.262,p= 0.006). A post hoc pairwise comparison using the Bonferroni correction showed increased OPT values between the baseline and 1 month follow-up assessments (4.4 vs 6.9, respectively), but this was not statistically significant (p= 0.152). However, the increase in OPT values did reach significance when comparing the baseline to the 3-months follow-up assessment (4.4 vs 7.2, p = 0.035). Therefore, results from the ANOVA indicate a significant effect of PRP injection in olfactory function across time as measured by OPT. The same process was applied to VAS score with the ANOVA determining that VAS differed significantly across the three time points (F (2,16) = 12.923,p= 0.004). A post hoc pairwise comparison using the Bonferroni correction showed an increased VAS score between baseline and 1-month follow-up assessments (4.1 vs 6.2, respectively), but this was not statistically significant (p= 0.075). Nevertheless, the VAS score increment reached significance when comparing the baseline to the 3-months follow-up assessments (4.1 vs 8, p = 0.008). Therefore, results from the ANOVA indicate a significant effect of PRP injection on olfactory function across time as measured by VAS score. Regarding complications, only one case of vasovagal episode was noted during the procedure, with no incurring cases of epistaxis or septal perforation during the 3-months´ follow-up.
Discussion
COVID-19 pandemic reinforced the pertinency of furtherly exploring the enigmatic world of olfactory science. To date, it is unknown why some COVID-19 patients experience pCIOD whereas others restore function shortly after 13. As pCIOD can significantly affect a patient´s quality of life, there is a need for effective treatment solutions 1). In this regard, PRP restores optimism by introducing a new potential treatment alternative.
This study´s primary purpose was to determine PRP injection´s efficacy, safety and feasibility in treating pCIOD. The primary objective of this work was met. Both the psychophysical (OPT) and subjective (VAS) olfaction scores exhibited significant improvement from the baseline to the 1-month and 3-months post-injection evaluations. These findings are in line with recent Literature. In an innovative pilot study published in 2020, Yan et al. first introduced the concept of applying PRP to manage long-lasting olfactory dysfunction 9. The results showed significant improvement in olfactory function in all patients receiving PRP treatment, but included a limited sample of 7 patients 9. The same group published the first randomized trial results of PRP application against placebo in pCIOD patients in late 2022, with favorable outcomes, mainly noted on olfactory discrimination 10. Meanwhile, other groups contributed significantly with larger PRP injected cohorts. Heba A. Abo El Naga et al. 14 focused on the effect of PRP injection in parosmia showing a highly significant improvement in VAS score. Lechien et al. 15 addressed pCIOD patients with anosmia, hyposmia and parosmia concluding that both psychophysical and subjective measurements were improved after injection. Interestingly, Duffy et al. refer to an alternative method of delivering PRP into the olfactory mucosa by means of PRP impregnated surgifoam instead of submucosal injection 16.
Another objective of this work was to describe possible PRP injection complications. In line with Literature 9,10, our protocol had a low rate of minor complications, with only one case of vasovagal reaction. No major complications occurred, including no cases of early/late epistaxis or resulting septal defects. Our unique single-site injection protocol contrasts with the one from Yan et al. 9, in which two different needle entry points are employed (the first along the superior septum just posterior to the head of the middle turbinate and then again about 1 cm posteriorly into the septum across from the leading edge of the superior turbinate). Conversely, in our single-site injection protocol, the puncture is only performed anteriorly, where the entry site can be controlled in the case of epistaxis (see Figure 3). Besides, virtually delineating three middle turbinate levels may help to avoid puncturing dangerously near to the S-point (see Figure 3). The authors believe this modification may somehow decrease the risk of incidental arterial puncture. On the other hand, the single-site injection allows for the PRP to dissect submucosally in a posterior and superior direction and deposit in situ without shunting through a second puncture point. Aside from ideal endoscopic accessibility in the awake patient, theoretical background seems to exist to support this anatomical site of injection and submucosal dispersion of PRP. The work from Escada 17 in cadaver specimens found that in the nasal septum, the lower limit of the olfactory mucosa lies at 15.9 ± 3.2 mm, 15.3 ± 3 mm and 16 ± 2.8 mm from the skull base, respectively at the anterior, middle and posterior portions of the olfactory region. Even though varying with individual anatomy, these measurements frame most of our procedures within the olfactory mucosa region (see Figure 5).
Another important modification from our protocol, compared to the one used in the randomized trial from Yan et al.9 was the injection timing. Yan et al 9 excluded patients with pCIOD lasting more than 12 months, since the authors believe neuronal regeneration is unlikely to occur after this period. Our cohort showed a mean of 6.5 months delay from COVID-19 infection and first specialized consultation. Additionally, one of our inclusion criteria was medical treatment failure measured by at least two consecutive 3-month apart OPT evaluations and pCIOD lasting for ≥ 12 months. Consequently, our mean time from COVID-19 diagnosis to PRP injection was 21 months. Apart from proving effectiveness in this setting, our findings showed that the time from COVID-19 diagnosis to injection did not associate with treatment response rates. In fact, the lack of correlation between injection timing and degree of improvement had similarly been reported in the randomized trial 10. Moreover, it is known that spontaneous recovery in post-viral olfactory dysfunction can potentially occur for longer than one year 18. Therefore, we believe that PRP treatment should also be offered to patients with longer lasting dysfunction. In addition, our results showed only mild or no procedural discomfort (VAS score ≤ 2) in all patients. Nevertheless, in our experience, despite being reasonably painless, the fear of anticipation makes it impactful in some patients. Hence, instead of using an empirical cycle of three separate injections within 2-week intervals 10, we prefer to perform only one injection with ulterior re-assessment.
This work also unveiled a peculiar mismatch between the VAS and OPT scores at 1 and 3 months, since the correlation between objective and subjective measurements was only significant at baseline. This had been previously observed in our pCIOD medical treatment protocol 1 and was somewhat described in the PRP pilot study 9. Analyzing Figure 4, one can note that a more substantial VAS score improvement was reached at the 3 months assessment. This may reflect the delay between the peripheral neuronal regeneration and central cortical integration. Alternatively, it may imply that an individual's everyday smell dysfunction experience has subtleties that our olfactory test battery cannot fully capture.
PRP is being used more frequently in many other surgical specialties 19. Concerning Otorhinolaryngology, PRP application is not limited to the olfactory science, as it has previously been suggested as beneficial in nasal surgery 20-22, otology 23 and cleft palate surgery 24. The exact mechanisms of how PRP may improve olfactory function are not fully understood. Some suggest that the high concentration of platelet derived molecules may stimulate the regeneration of olfactory neurons, improve the function of the olfactory epithelium and halt the inflammatory burden 9,14. However, studies are limited by small sample sizes, heterogeneous patient populations, and PRP preparation and administration variations. Therefore, more extensive and well-designed studies are needed to confirm these findings and establish optimal treatment protocols.
This study has its limitations. A drawback is the absence of randomization and placebo arm in research with few patients. We acknowledge that a control group would be ideal for determining PRP effectiveness and ruling out the placebo effect. It should also be noted that our PRP process included a low dose of sodium citrate as an anticoagulant for PRP purification. Sodium citrate has been proven to have good platelet recovery and mesenchymal stromal cell proliferation compared to other anticoagulants, making it an ideal anticoagulant. However, some research suggests that sodium citrate can itself ameliorate olfactory impairment in postinfectious olfactory loss 25. In this way, there might be a synergic effect of PRP when combined with sodium citrate yielding a 3- to 5-fold increase in growth factor production 9. Thus, this fact could have influenced the results. Also, for convenience, only OPT was measured in the psychophysical domain, so that identification and discrimination were not evaluated in this sample. Also, in order to homogenize the sample, only pCIOD patients were included, although the initial pilot study of PRP injection included other etiologies of olfactory dysfunction 9. Our study has its own strengths as well. It is the first to describe an effective single-site PRP injection protocol applied to olfactory dysfunction. Also, it describes therapeutic efficacy of PRP injection in patients suffering from pCIOD for ≥ 12 months.

Figure 5 Estimated vertical distribution of olfactory mucosa at the anterior olfactory septal portion (white line), based on Escada´s cadaveric measurements 17. Green dashed lines correspond to the virtual vertical divisions of the middle turbinate. In this case, the injection site was by far inside the olfactory epithelium.
Conclusion
We comprehensively described a new single-site PRP injection protocol for olfactory dysfunction management. The olfactory outcomes of such intervention on pCIOD patients were explored. In line with very recent Literature, our results suggest that PRP injection into the olfactory mucosa is an innovative, safe and effective approach to improve olfactory recovery. Therefore, this study reinforces the determinant role of the Otorhinolaryngologist in the treatment of olfactory complaints. Nevertheless, despite promising results, further research is needed to confirm the effectiveness of PRP in olfactory mucosa regeneration, and to establish optimal protocols for PRP preparation and injection technique. More studies with larger sample sizes and extended follow-up periods could help to fully understand its potential benefits and to standardize the treatment approach.
Acknowledgments
We want to thank Dr. André Varandas Borges from the Physical Medicine and Rehabilitation Department of Centro de Reabilitação do Norte who promptly transmitted his knowledge about PRP usage into our practice. Also, to Dr. João Carvalho Almeida from the Otorhinolaryngology Department of Centro Hospitalar Universitário de Santo António for his knowledgeable advices, support of the work, and aid in graphic illustration. We would also like to express the deepest gratitude towards the consultation head nurse Paula Lamas and her team for the unwavering support, so as to various colleagues from the authors´ Department who assisted the interventions. We are profoundly thankful to Dra. Cecília Almeida e Sousa for her guidance and support since the early beginning of our smell and taste consultation project.
Conflict of Interests
The authors declare that they have no conflict of interest regarding this article.
Data Confidentiality
The authors declare that they followed the protocols of their work in publishing patient data.
Human and animal protection
The authors declare that the procedures followed are in accordance with the regulations established by the directors of the Commission for Clinical Research and Ethics and in accordance with the Declaration of Helsinki of the World Medical Association.


















