Introduction
Functional endoscopic sinus surgery (FESS) is a commonly performed surgical procedure to treat chronic sinusitis and other sinonasal disorders. One of the major challenges in FESS is the management of bleeding 1,2. Intra-operative bleeding causes considerable loss in visual perception during surgery 2 . Since the nasal cavity is a narrow space with abundant blood supply 2, blood can obscure the anatomy of the operating field and stain the endoscope lens, compromising visibility 1. This fact may increase the risk of surgical complications, lengthen the operatory time, yield poorer surgical results or even prohibit the completion of the surgery 1,2.
Some strategies have been tried to ameliorate operating conditions in FESS 1. Tranexamic acid (TXA) and hot saline irrigations (HSI) are two potential interventions to control bleeding and improve the surgical field quality in this setting 3-14. Tranexamic acid is a synthetic amino acid that works by inhibiting fibrinolysis. It is commonly applied to reduce bleeding and transfusion requirements. In FESS, TXA has been shown to reduce operative time and complications while increasing surgeon´s satisfaction 3,4,7-14. On the other hand, HSI is a simple and inexpensive intervention that involves using warm saline irrigations to increase blood vessel constriction and reduce bleeding 1,15. Studies have demonstrated that the use of HSI in FESS can significantly reduce operative time and complications by improving the quality of the surgical field 3,4,7,15.
Overall, TXA and HSI have both been shown to be useful adjuvants in FESS, without any deleterious effect on intraoperative hemodynamic stability. These interventions have been shown to be safe and effective and could be considered part of the standard of care for FESS in the near future 2,15-19. Nevertheless, no research to date compares these two modalities comprehensively. In this regard, the main objective of this work was to aggregate evidence concerning the effects of TXA and HSI on blood loss, surgical field visibility and duration of surgery in FESS.
Material and Methods
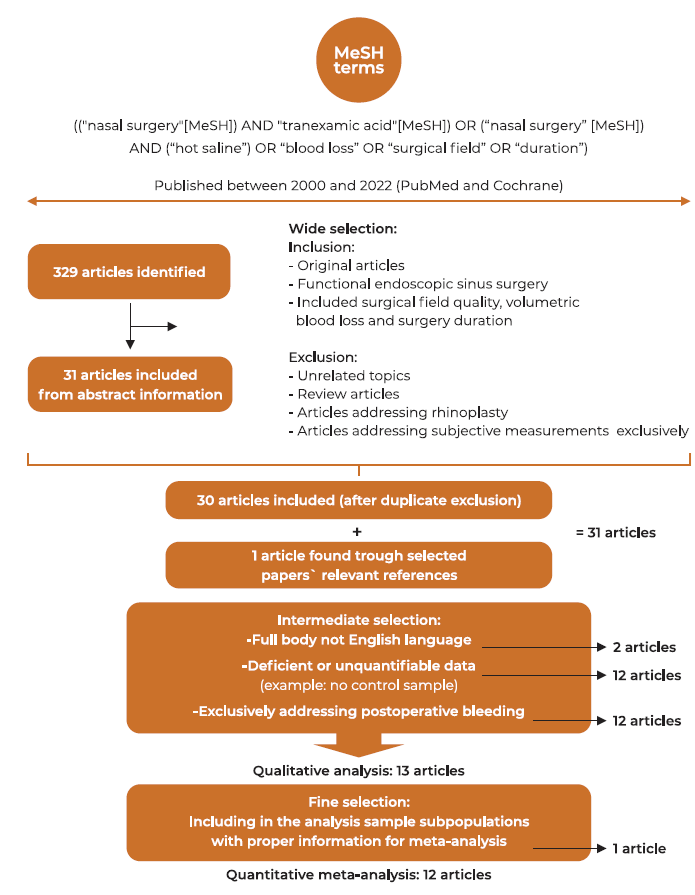
The review procedure was based on the criteria for Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. One can find the summary of the review process in Figure 1.
Search strategy
A database search spanning records from January 2000 to December 2022 was conducted by two Investigators (FS and MS) across PubMed and the Cochrane Library. Two separate types of investigations were performed in parallel. One, identified studies relating to FESS and TXA, while the other looked for works relating to FESS and HSI. A step-by-step approach was used to gather original results on FESS related outcomes. The search was limited to papers published in English language. The primary selection was made using any combinations of the following terms: "nasal surgery,” tranexamic acid," “hot saline,” “blood loss”, “surgical field,” and/or “duration”. Bibliographies of the included studies were manually checked to find additional relevant literature. Finally, the explorative results from the two investigators were matched to bring the final research pool.
Selection process
Relevant literature was selected in three main phases (Figure 1). In the wide selection phase, exclusion and inclusion criteria were applied after abstracts´ comprehensive analysis. Articles addressing unrelated topics or exclusively covering subjective blood loss measurements were excluded. In the intermediate selection phase, the article's complete body information was analyzed to evaluate inclusion and perform a qualitative analysis. The final sample to be integrated into the quantitative analysis was obtained during the fine selection phase. At any of the steps, the paper was disregarded if found to incur any of the exclusion criteria (Figure 1).
Outcomes
The three main outcomes were: volumetric bleeding loss (vBL), surgical field quality (SFQ) (also known as intraoperative bleeding score), and duration of surgery (DS). Intra-operative vBL was measured by the amount of blood in the suction collector and by measuring postoperative weight of dressings. SFQ was graded in terms of bleeding by means of the Wormald 20 and Boezaart 21 grading scales. DS was registered in minutes.
Assessment of risk of systematic bias
We assessed the methodological quality of the included studies and carried out the assessment of risk of bias, taking into consideration: method of randomization; allocation concealment; blinding; incomplete outcome data; selective outcome reporting and overall. We used the Version2of the Cochrane risk-of-bias tool for randomized trials (RoB2), which involved describing each of these domains as reported in the trial and then assigning a judgment about the adequacy of each entry as low, high, or unclear risk of bias. We presented this information in a ‘risk of bias’ summary.
Statistical analysis
Meta-analysis was carried out in compliance with Cochrane Collaboration standards using RevMan v5.4 (Cochrane, London, United Kingdom). A composite effect size estimate was used to reflect relevant measures in the meta-analysis. For meta-analytical comparison, the effect size of each measure, as well as the 95 percent confidence interval and heterogeneity as defined by the I2 statistic, were employed. Means and standard deviations were obtained through descriptive statistics, with statistical significance set at p < 0.05. The outcomes of patients from studies that precisely reported the outcome of interest were compared. A random effects model was utilized to calculate the effect sizes. Each branch was treated as a unique study for studies with several research branches. Standardization was based on the standardized mean difference using Cohen's d values for studies reporting continuous variables.
Results
Descriptive results
Three hundred and twenty-nine records were found in the primary search. Titles and abstracts were reviewed, and 298 papers were excluded. 31 papers were comprehensively evaluated for pertinency. After thoroughly reviewing the article's whole body, 18 articles were removed. 13 studies were used in the qualitative analysis (Table 1). Using the RoB2, papers were assessed for risk of bias. Following quality assessment, 8 papers were graded as low risk, 4 as having some concerns and 1 paper was graded as high risk of bias (see Table 2 for details). The research by Athanasiadis et al. was the only ‘intra-individual study design’ in the quantitative analysis 8, with the other studies assigning individuals to either an intervention or placebo-controlled arm. Eldaba et al. was the only work to include a pediatric population 14. The study from Chhapola & Matta 22 was excluded from the meta-analysis because statistical data was not adequately reported. The study from Shehata et al 3 compared HSI and TXA and therefore provided both HSI and TXA sample´s subgroups.
A total of twelve papers were therefore included in the meta-analysis: two relating to HSI 4,7, nine relating to TXA 5,6,8-14, and one comparing TXA and HSI 3. All were prospective randomized controlled studies. A total of 106 HSI patients and 357 TXA patients were included (463 cases) and matched with controls (n = 412), making for a total of 875 patients. Of the ten articles which included TXA samples, intra-operative TXA was used either intravenously 6,9,10,12-14 or topically 3,5,8,11. Figure 1 displays the flow diagram of the selection.
Concerning primary outcome measures, vBL was covered in eleven studies 3-7,9-14, SFQ in ten studies 3,4,7-14, and DS in seven studies 3,4,7,9,12-14. Among the 12 included studies in the meta-analysis, 11 reported significant improvements in the outcomes of interest in cases against control groups (91.2%).
Table 1 Summary of the studies included in the systematic review.
| Study | Sample size of interest | Type of intervention | Control Comparator | Administration period | Population | Relevant outcomes | Pertinent findings |
|---|---|---|---|---|---|---|---|
| Shehata et al 2014 [3] ‡ | 75 (25 HSI 25 TXA 25 controls) | HSI (50ºc) Topical TXA - 1 g in 20 ml saline | NS (20ºc) | Intraoperative packing and irrigation | Adults (Age 20-50 years) | -vBL -SFQ -DS -SS - MAP | The use of local TXA and HSI up to 50°C achieved reduction in vBL, DS and improved SFQ during FESS, without impact on MAP. |
| Al-Issis et al 2016 [7] ‡ | 50 cases 50 controls | HSI (48ºc) | NS (20ºc) | Intraoperative irrigation | Adults (Age 28-58 years) | -vBL -SFQ -DS -SS -MAP | Significant decrease in BL and DS with improved SS and SFQ in HSI sample. |
| Gan et al 2003 [4] ‡ | 31 cases 31 controls | HSI (49ºc) | NS (18ºc) | Intraoperative irrigation | Adults (Age≥ 19 years) | vBL -SFQ -DS -MAP - HR | HSI improves SFQ after 2 hours of operating time. Significant reduction in rate of vBL with HSI. |
| Jabalameli & Zakeri 2006 [5] ‡ | 26 cases 30 controls | Topical TXA - 1 g in 20 ml saline | 20 ml of NS | Intraoperative (when target MAP was reached) *1 | Adults (Age 18-55 years) | -vBL -SFQ | The bleeding score of TXA group was significantly lower than of placebo group |
| Langille et al 2013 [9] ‡ | 14 cases 14 controls | Intravenous TXA Bolus:15 mg/kg Infusion: 1 mg/kg per hour | NS bolus and infusion | Pre-operative bolus + Intra-operative infusion | Adults (Age 23-80 years) | -vBL -SFQ - DS -MAP -Lund-Kennedy score -POSE score *2 --ETCO2 | Adjunctive TXA does not appear to result in a clinically meaningful reduction in vBL or improve SFQ during FESS. |
| Dongare & Saundattikar 2017 [10] ‡ | 30 cases 30 controls | Intravenous TXA Bolus: 15 mg/kg | NS bolus | Pre-operative | Adults (age range NR) | -vBL -SFQ -DS -MAP -ETCO2 | TXA has a beneficial role in FESS by improving the SFQ when used as an adjunct. |
| Jahanshahi et al 2014 [11] ‡ | 30 cases 30 controls | Topical TXA (Three pledgets soaked in 5% TXA and 0.5% phenylephrine for 10 minutes) | three pads soaked with 0.5 % phenylephrine for 10 minutes | Pre-operative | Adults (age 18-60 years) | -vBL -SFQ | -Topical TXA can efficiently reduce vBLand improve the SFQ in FESS patients with rhinosinusitis. |
| Eldaba et al 2013 [14] ‡ | 50 cases 50 controls | Intravenous TXA 25 mg/kg in 10 ml NS - slow injection 3-5 min | Intravenous 10 ml NS slow injection 3-5 min | Pre-operative | Children (age mean around 7 years, range NR) | -vBL -SFQ -DS -MAP -HR | Single intravenous bolus dose of TXA in children during the FESS improves SFQ and reduces both vBL and DS. |
| Alimian & Mohseni 2011[13] ‡ | 42 cases 42 controls | Intravenous TXA 10 mg/Kg bolus | Intravenous sterile water | Pre-operative | Adults (age 19-64 years) | -vBL -SFQ -DS -MAP | Intravenous TXA effectively reduces bleeding and improves the SFQ during FESS. |
| Nuhi et al 2015 [6] ‡ | 100 cases 70 controls | Intravenous TXA 15 mg/Kg bolus | NS bolus | NR (assumption of pre-operative bolus) | Adults (age range NR) | -vBL -SFQ -HR | Intravenous TXA decreased vBL and need for antihypertensive agents, without increasing side effects. |
| Athaniasiadis et al 2007[8] ‡ | 10 cases 10 controls | Topical spray using microatomiser 100 mg TXA | Topical spray using microatomiser NS | NR | Adults (Age range 19-79) | vBL -SFQ -SS -MAP -HR -ETCO2 | Topical application of TXA is effective in achieving hemostasis and improving the surgical field. |
| El Shal & Hasanein 2014 [12] ‡ | 30 cases 30 controls | Intravenous TXA 10 mg/Kg in 100 ml saline administered during 10 min infusion | Intravenous 100 ml NS injection 10 min | Pre-operative | Adults (Age range 18-50 years) | vBL -SFQ -SS -DS -MAP -HR | Intravenous TXA effectively reduce vBL during FESS and improve SFQ and SS. |
| Chhapola & Matta 2011 [22] ¤ | 100 cases 100 controls | Intravenous TXA infusion (500mg in 100ml normal saline) 20-30 minutes preoperatively | Did not receive TXA | Pre-operative | Adults (Age range 18-58 years) | -vBL | TXA decreases vBL by 72.48% even in absence of hypotensive anesthesia and irrespective of the type of anesthesia used. |
HSI: Hot saline irrigations; TXA: Tranexamic acid; NSI: Normal saline (Room temperature saline); FESS - functional endoscopic sinus surgery; MAP - Mean arterial pressure; vBL - volumetric blood loss; DS - duration of surgery; SFQ -surgical field quality; SS - surgeon´s satisfaction; HR - Heart rate; *1 target MAP: defined as 30% below patient’s preoperative MAP in this study; *2 Peri-Operative Sinus Endoscopy (POSE) score; NR - Not reported; ETCO2- end tidal CO2; ‡: included in the meta-analysis; ¤ : excluded from the meta-analysis
Table 2 Individual Randomized Controlled Trial Methodological Quality
| Study | Random sequence Generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Free of selective reporting (reporting bias) | Risk of bias of randomized studies (overall) |
| Shehata et al 2014 [3] | ? | + | + | + | + | + | + |
| Al-Issis et al 2016 [7] | + | ? | - | ? | ? | ? | ? |
| Gan et al 2003 [4] | + | + | + | + | + | + | + |
| Jabalameli & Zakeri 2006 [5] | ? | + | + | ? | + | + | ? |
| Langille et al 2013 [9] | + | + | + | + | + | + | + |
| Dongare & Saundattikar 2017 [10] | ? | + | + | + | + | + | + |
| Jahanshahi et al 2014 [11] | + | + | + | + | + | + | + |
| Eldaba et al 2013 [14] | + | + | + | + | + | + | + |
| Alimian & Mohseni 2011[13] | + | + | + | + | + | + | + |
| Nuhi et al 2015 [6] | + | + | + | + | ? | ? | ? |
| Athaniasiadis et al 2007[8] | + | + | + | + | ? | ? | ? |
| El Shal & Hasanein 2014 [12] | + | + | + | + | + | + | + |
| Chhapola et al 2011 [22] | ? | - | - | ? | ? | ? | - |
Green with plus symbol = low risk; yellow with question mark = some concerns; red with minus sign = high risk
Meta-analysis
Blood loss
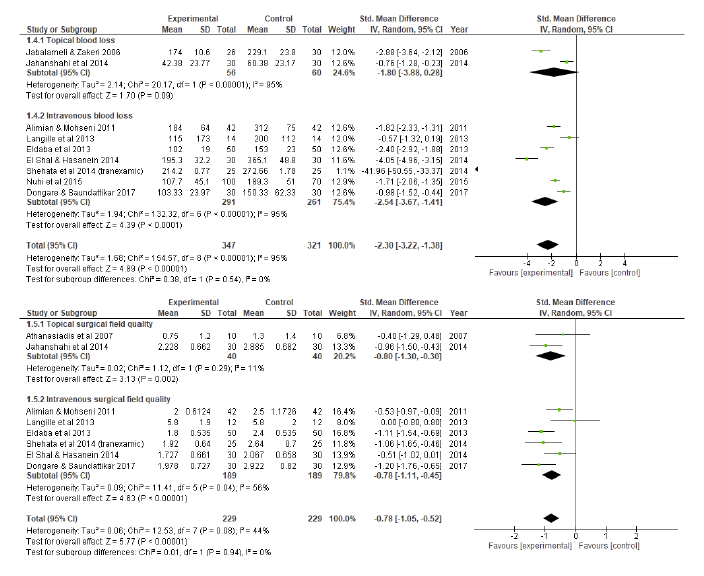
A random effects model of standard mean differences showed that patients undergoing HSI or TXA experienced significant reduction in vBL compared to controls, yielding a combined effect size of - 2.82 (p < 0.001) (Figure 2). Individually, both HSI (p<0.001) and TXA (p<0.001) were significantly superior to controls in reducing vBL. Considering the subgroup analysis (Figure 2), HSI presented a more significant reduction in vBL than TXA (effect size of HSI: - 7.55 vs effect size of TXA: - 2.30, p = 0.01).
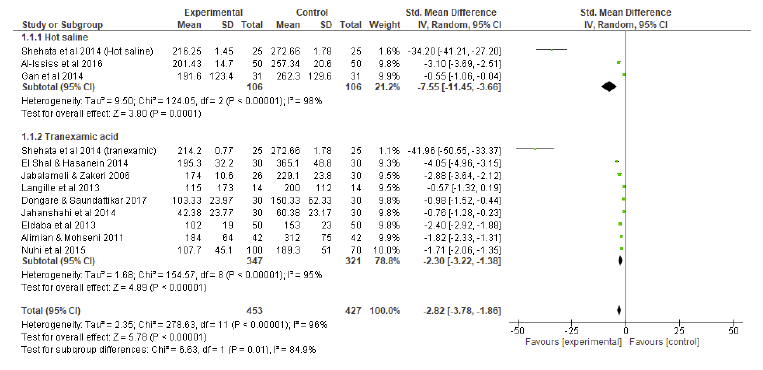
Fig 2 Blood loss compared to controls: subgroup analysis forest plot. SD = standard deviation; IV = inverse variance; CI = confidence interval
Surgical field quality
A random effects model of standard mean differences showed that patients undergoing HSI or TXA experienced significant improvement in SFQ compared to controls, yielding a combined effect size of - 0.78 (p < 0.001) (Figure 3). Individually, both HSI (p<0.001) and TXA (p<0.001) were significantly superior to controls in terms of SFQ. Considering the subgroup analysis (Figure 3), no differences were found regarding SFQ between HSI and TXA (effect size of HSI: - 0.78 vs effect size of TXA: - 0.78, p = 0.99).
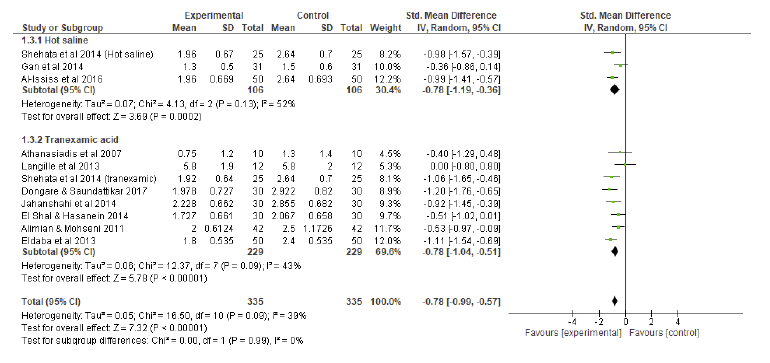
Fig.3 Surgical field quality (intraoperative bleeding score) compared to controls: subgroup analysis forest plot. SD = standard deviation; IV = inverse variance; CI = confidence interval
Duration of surgery
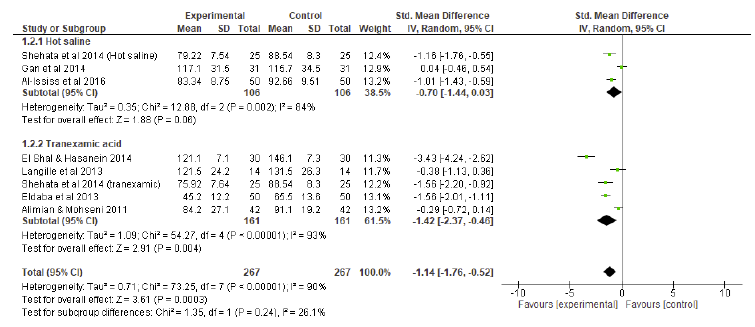
A random effects model of standard mean differences showed that patients undergoing HSI or TXA experienced a significant reduction in DS compared to controls, yielding a combined effect size of - 1.14 (p < 0.001) (Figure 4). Individually, although showing some tendency for significance, HSI did not show a significant reduction in DS compared to controls (p=0.06). TXA was significantly superior to controls in terms of DS (p=0.004). Considering the subgroup analysis (Figure 4), no differences were found regarding DS between HSI and TXA (effect size of HSI: - 0.70 vs effect size of TXA: - 1.42, p = 0.24).
Topical versus intravenous tranexamic acid
An additional subgroup analysis was performed to compare the effects of topical versus intravenous TXA on vBL and SFQ (Figure 5). DS was not computed since no topical TXA studies sufficiently reported this outcome. A random effects model of standard mean differences showed that patients undergoing both topical and intravenous experienced a significant reduction in vBL (combined effect size of - 1.38, p < 0.001) and SFQ (combined effect size of - 0.78, p < 0.001). No differences were observed between topical and intravenous TXA concerning v BL (p = 0.54) or SFQ (p = 0.94).
Discussion
There is a close association between intraoperative bleeding and surgical field quality in FESS 15. Adequate visualization during FESS is critical for total disease eradication and avoiding complications 1,2,15. When SFQ is compromised, orbital or skull base iatrogenic injuries may ensue 15. Additionally, intra-operative bleeding causes multiple interruptions during surgery for suctioning and packing, which may increase surgical time. Hence, exploring adjuvant therapeutics to maximize operatory conditions is essential.
This study´s primary purpose was to compare HSI and TXA regarding vBL, SFQ, and DS. The primary objective of this work was met. HSI presented a more significant reduction in vBL compared to TXA. Considering SFQ, both HSI and TXA were significantly superior to controls, and no significant difference was found between HSI and TXA. Concerning the DS, there were no significant DS differences between HSI and TXA patients. Nevertheless, only in the TXA group DS was significantly reduced compared to controls (probably due to a larger sample size). The only existent study directly comparing HSI and TXA is the one from Shehata et al 3. In this trial, three groups, TXA 1000 mg diluted in 20 ml saline, 50ºc HSI, and normal saline were used for packing and irrigation during FESS. The use of local TXA and HSI up to 50°C achieved a significant reduction in vBL and DS, while improving SFQ. Notably, no significant differences were observed between TXA and HSI groups. These findings are partially in line with our results. Although we found a significantly reduced vBL in the HSI group compared to the TXA, this did not translate into higher SFQ or reduced DS.
Consistent with our findings, a previous meta-analysis reported that the use of HSI provides a good hemostatic effect during FESS to control intraoperative bleeding and obtain superior SFQ 15. HSI were first used to alleviate epistaxis 23, with the advantage of being less uncomfortable and damaging to the nasal mucosa than nasal packing 24. The hemostatic mechanism of HSI is unknown, but it may include edema and narrowing of the intranasal lumen, which contributes to vessel compression; decreased flow and intraluminal blood pressure due to mucosal vasodilation; or cleaning of blood coagulates from the nose 25. The resulting decrease in diffuse mucosal oozing is pointed out as one important aspect of SFQ improvement with HSI 26. Another key advantage of HSI is that it allows the cleaning of the endoscopic lens 1,3. Local acting effects could help to explain why HSI proved superior to TXA in reducing vBL in our study. In HIS there is always local administration, contrasting to systemic administration in many TXA studies. We wonder if this fact could potentiate a more localized modulation of bleeding mechanisms. On the other hand, the comparison between topical versus intravenous TXA administration did not show significant differences between groups concerning vBL and SFQ, since both were seemingly effective on ameliorating these outcomes.
With inherent antifibrinolytic action, TXA acts by competitive binding with the lysine site on plasminogen 3. This reduces bleeding by preventing fibrinolysis and stabilizing blood clots. In line with our results, former meta-analyses 2,16-19 concluded the effectiveness of topical and systemic TXA in reducing blood loss and improving SFQ in FESS. Likewise, in our systematic review, most of the studies showed favorable outcomes with the use of TXA. Only the study from Langille et al 9 failed to show a beneficial association between TXA and intraoperative bleeding in FESS. When scrutinizing the results of that same study 9, one can find that there was a median of 115 ml blood loss in cases versus 200 ml in controls, and the median DS was 10 minutes shorter in the TXA group. Hence, neither vBL nor DS reached significance probably due to a limited sample size of 14 patients, which was too small to accommodate significance.
In our study, TXA showed a significant shorter DS compared to controls, opposing to HSI in which significance was not reached. We wonder if this is explained by: 1) TXA administration not being dependent on the surgeon (when intravenous administration is used) or 2) TXA being applied only once in a while when topically, as opposed to HSI, which requires multiple repeated administrations throughout the surgery, making it more time-consuming.
TXA´s most prevalent side effects are gastrointestinal, including postoperative nausea and vomiting. However, the incidence of these side events is modest 18. A rapid bolus dose might produce substantial hypotension when administered intravenously 27. Nevertheless, recent studies show that adverse effects of TXA are dose-dependent and infrequent at the recommended dosages 18. Many of the enrolled trials used safe administration techniques such as gradual injection and safe dosages between 10-15 mg/kg. Despite TXA´s safety and tolerance, the possibility of thromboembolic events has raised concerns. Nevertheless, current studies demonstrate that TXA does not significantly increase the incidence of thromboembolism when compared to controls 18.
This study has limitations. As with every systematic review and meta-analysis, there is a certain risk of publication bias. Moreover, the included studies differ in their design and methodologies, resulting in considerable heterogeneity in reports. There are several surgical approaches within FESS, and the experience of surgeons and baseline pathology may have varied between cases and controls, as this is not always reported. Besides, even with proper preoperative coagulation and platelet count tests (performed in most studies) it is impossible to account for the subclinical and interindividual susceptibility to bleeding, which may have compromised the analysis. Overall, results should be appraised critically as a result of research heterogeneity. Our study has its own strengths, as it is the first meta-analysis to compare HSI and TXA effectiveness in bleeding-related outcomes in FESS.
Conclusion
Bleeding during FESS continues to be a problem for both surgeons and anesthesiologists. This study suggests that the use of HSI or TXA in FESS can improve operative conditions while reducing blood loss. Both are effective in reducing blood loss (with a marginal advantage in HIS in vBL), and no differences were found between the two modalities relating to SFQ and DS. TXA has the advantage of being easy to apply, especially when used intravenously. HIS on the other hand may help to clean the endoscope lens and remove blood clots from the field. Studies focusing on the synergic effect of applying both TXA and HSI could also be relevant to affirm their role in daily practice.
Conflict of Interests
The authors declare that they have no conflict of interest regarding this article.
Data Confidentiality
The authors declare that they followed the protocols of their work in publishing patient data.
Human and animal protection
The authors declare that the procedures followed are in accordance with the regulations established by the directors of the Commission for Clinical Research and Ethics and in accordance with the Declaration of Helsinki of the World Medical Association.