Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.21 no.6 Lisboa dez. 2014
https://doi.org/10.1016/j.jpge.2014.07.002
CLINICAL CASE
Chronic intestinal pseudo-obstruction as an expression of inflammatory enteric neuropathy
Pseudo-obstrução intestinal crónica: uma manifestação de neuropatia entérica inflamatória
Rita Pimentela,∗, Marta Salgadoa, Maria João Magalhãesa, Ana Accarinob, Isabel Pedrotoa
a Gastroenterology Department, Centro Hospitalar do Porto, Hospital de Santo António, Porto, Portugal
b Gastroenterology Department, Hospital Universitari Vall dHebron, Barcelona, Spain
* Corresponding author.
ABSTRACT
Background Chronic intestinal pseudo-obstruction (CIPO) is characterised by inadequate digestive tract motility and can lead to severely disordered motility. CIPO manifests as recurrent episodes of intestinal sub-occlusion without an anatomical obstruction. We present the case of a 41-year-old female, with severe chronic constipation and several episodes of intestinal sub-occlusion. Investigation revealed colonic inertia and marked distension of the small bowel and colon with no evidence of stenosis or obstructive lesions, compatible with CIPO. After several treatments were tried (domperidone, erythromycin, cisapride, octreotide, total enteral nutrition), with partial or no response, further work-up was done trying to identify an etiology. Gastrointestinal manometry showed neuropathic type abnormalities, transmural biopsy of the jejunum revealed degenerative enteric neuropathy and anti-HU antineuronal antibody screen was positive, suggesting an autoimmune type neuropathy with diffuse involvement of the digestive tract. Corticosteroids showed partial improvement of short duration and azathioprine was also tried but discontinued due to intolerance. Marked dietary intolerance and malnutrition lead to total parenteral nutrition (TPN) at home since October 2011. Since then, symptoms and nutritional status improved, with rare episodes of pseudo-obstruction, not requiring hospitalisation.
Keywords: Chronic intestinal pseudo-obstruction; Neuropathy; Bowel
RESUMO
Pseudo-obstrução intestinal crónica (POIC) caracteriza-se por motilidade inadequada do tubo digestivo, frequentemente com dismotilidade severa. Manifesta-se por episódios recorrentes de suboclusão intestinal, sem obstrução anatómica. Apresentamos o caso de uma mulher de 41 anos, com obstipação crónica severa e episódios de suboclusão intestinal. A investigação inicial revelou inércia cólica e distensão marcada do intestino delgado e cólon, sem lesões obstrutivas, compatível com POIC. Após diversas terapêuticas (domperidona, eritromicina, cisapride, octreótido, nutrição entérica total),com eficácia parcial ou nula, progrediu-se na investigação tentando esclarecer a etiologia. A manometria gastrointestinal revelou alterações neuropáticas e uma biopsia transmural jejunal demonstrou alterações degenerativas entéricas. Estes achados, associados á presença de anticorpos antineuronais anti-HU, sugeriam uma neuropatia entérica autoimune atingindo difusamente o tubo digestivo. Com corticoterapia obteve-se melhoria parcial e de curta duração e azatioprina foi tentada mas com intolerância a obrigar á sua descontinuação. Devido ao desenvolvimento de intolerância alimentar marcada, associada a desnutrição, a doente está sob nutrição parentérica total domiciliária desde 10/2011, com melhoria significativa dos sintomas e estado nutricional, raros episódios de pseudo-obstrução, sem necessidade de internamentos.
Palavras-Chave: Pseudo-obstrução intestinal crónica; neuropatia; Intestino
1. Introduction
Chronic intestinal pseudo-obstruction (CIPO) is a disorder characterised by inadequate digestive tract motility, generally due to poor neuromuscular function, which can lead to severely disordered gastrointestinal motility.1 The condition manifests itself as recurrent episodes of intestinal subocclusion, clinically similar to mechanical obstruction, but without the presence of an anatomical obstruction.2 It is caused by a disturbance in the enteric nervous system or extrinsic nervous system or by a disorder of the smooth muscle and may be a primary disorder (visceral neuropathy or visceral myopathy) or secondary to another systemic disorder.3 Oftentimes the aetiology cannot be identified and is considered idiopathic CIPO. Diagnostic assessment includes excluding mechanical obstruction of the digestive tract, documenting dilatation of intestinal segments, determining whether other segments of the digestive tract besides the small intestine are involved and, if possible, determining the aetiology. Treatment is often inadequate but is aimed at improving symptoms and ensuring that the patients nutritional needs are adequately met.
2. Clinical case report
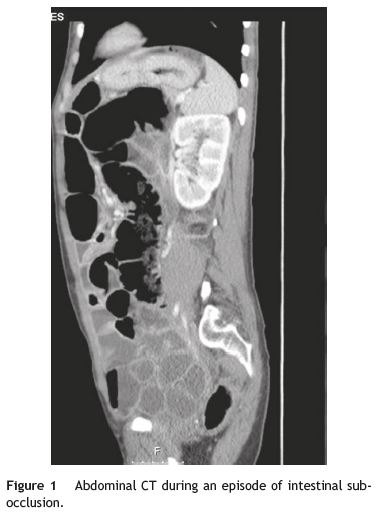
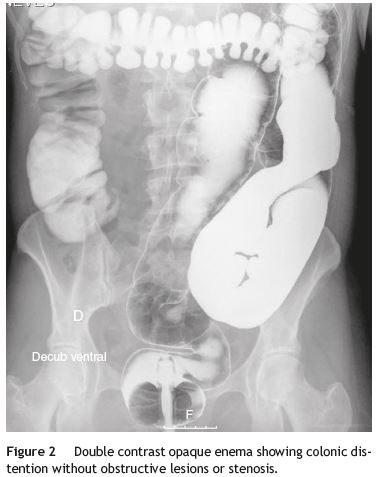
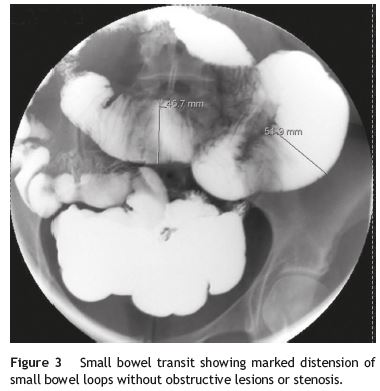
Our patient was a 41-year-old female with Raynauds phenomenon, with no relevant history or family history of digestive disorders. She had a history of severe constipation since the age of 29, with partial response to laxatives. Associated with the constipation, the patient experienced episodes of marked abdominal distension, abdominal pain, nausea and vomiting. Abdominal CT done during one of these episodes revealed marked distension of the colon with no evidence of obstruction (Fig. 1). Further work-up done since then showed: upper GI study was normal; small bowel transit and double contrast opaque enema studies showed marked distension of the jejunal loops and of the colon, with no evidence of stenosis or obstructive lesions (Figs. 2 and 3); gastric emptying scintigraphy was compatible with gastroparesis; colonic transit time was compatible with colonic inertia. Based on this clinical picture and the exam results, a diagnosis of CIPO was put forward. A rectal biopsy ruled out amyloidosis. The patient did not meet the criteria for conjunctive tissue disease. In 2007 (at 36 years of age), the patient began to experience dysphagia with solid foods and episodes of food impaction. Oesophagealgastro-duodenal transit showed reduced distensibility of the oesogastric junction, oesophageal manometry results were compatible with achalasia. The dysphasia resolved after endoscopic dilatation with a 30 mm Rigiflex balloon.
Beginning in 2009, the patients clinical status progressively deteriorated, with frequent episodes of sub-acute intestinal obstruction leading to multiple hospitalisations (she was in hospital nearly continuously over a period of 6 months in 2011), with a weight loss of 15 kg (BMI - 17 kg/m2) and malnutrition. She developed complete intolerance to regular food and was on total enteral nutrition for approximately 2 years. She did not improve with domperidone, had only a partial temporary response to erythromycin and later to cisapride, nor did she respond to octreotide. The hydro gen breath test was suggestive of bacterial overgrowth, so the patient was medicated with cycles of antibiotics, but showed no improvement. In 2011 the patient underwent gastrointestinal manometry, which showed neuropathic type abnormalities. An electromyogram with dysautonomia test showed no evidence of peripheral or autonomic neuropathy. A transmural biopsy of the jejunum, with immunohistochemical testing, revealed degenerative enteric neuropathy with oligoneuronal hypoganglionosis of the myenteric plexus; no changes were observed in the muscle layer. An anti-HU antineuronal antibody screen was positive, suggesting that we were dealing with an autoimmune type neuropathy (after paraneoplastic syndrome was ruled out) with diffuse involvement of the digestive tract. Although it is known that intestinal failure is invariably the final stage of CIPO, because of the findings suggesting autoimmunity and in an attempt to at least delay progression of the disease, the patient was treated with corticosteroids (methylprednisolone 100 mg/day IV for three days followed by oral prednisolone, with gradual tapering of the dose over a 3-month period). Partial improvement of short duration was observed. The patient was also started on azathioprine (1.5 mg/kg/day), but this was discontinued due to intolerance.
Marked dietary intolerance and malnutrition made it necessary to put the patient on total parenteral nutrition (TPN) at home, which she has continued since October 2011 with no complications thus far. The patients symptoms have improved, with rare episodes of sub-acute intestinal obstruction not requiring hospitalisation. Her nutritional status has also significantly improved and she has gained weight (current weight - 54 kg, BMI - 21 kg/m2).
3. Discussion
CIPO is the most severe form of dysmotility of the digestive tract, which is a failure of the intestinal contents to progress through the digestive tract.3 Advances in functional studies and histological analysis have enabled a better understanding of the pathogenesis of this syndrome, which is defined as an enteric neuromuscular disorder that frequently involves various segments of the digestive tract and not just the small intestine.4 Based on the pattern of dysmotility and histopathologic changes, the condition is classified as visceral neuropathy, mesenchymal disease (involvement of the interstitial cells of Cajal) or visceral myopathy.3 Visceral neuropathies can be inflammatory or degenerative. Inflammatory neuropathies can be idiopathic or secondary (e.g. infections, paraneoplastic syndrome) and are characterised by the presence of a dense lymphocytic infiltrate involving the myenteric plexus (ganglionitis) causing neuronal degeneration.5 In its most severe forms, complete depletion of ganglion cells may occur (aganglionosis).6 Enteric ganglionitis may occur throughout the digestive tract causing severe functional damage. Cases of CIPO have been described that were due to inflammatory neuropathy associated with the presence of anti-HU antineuronal antibodies, which points to an autoimmune mechanism.7,8 Screening for these antibodies is recommended for diagnosing intestinal dysmotility associated with enteric ganglionitis.5 Antineuronal antibodies may be asso ciated with a paraneoplastic syndrome but may also be present in cases of idiopathic CIPO. Anti-Hu antibodies are the most common type of antineuronal antibodies. The basis of pharmacological treatment is immunosuppression, using corticosteroids either alone or in combination with other immunosuppressants, such as azathioprine or cyclophosphamide, described with variable results.7,8 In the case of our patient gastrointestinal manometry revealed a pattern consistent with enteric neuropathy, which, in combination with the presence of antineuronal antibodies, suggested the possibility that we were dealing with an autoimmune enteric neuropathy (enteric ganglionitis). The histologic changes, however, showed degenerative neuropathy with oligoneuronal hypoganglionosis. These findings could be explained by a severe inflammatory process diffusely affecting the myenteric plexus that occurred in the past, which is no longer present but which led to severe degeneration with ganglion and neuron depletion. This hypothesis may explain the patients poor response to corticosteroids.
CIPO causes food intolerance, with inadequate food intake, and malabsorption, so malnutrition is one of the main problems and therefore nutritional support is a mainstay of the treatment. Except for the acute episodes of pseudo obstruction, when no oral intake is necessary for a short period, whenever possible oral feeding (small meals and/or enteral nutritional supplements) is advised, because is better tolerated and has less complications than total parenteral nutrition (TPN). TPN is reserved for the most severe cases (intestinal failure), as the only way to satisfy nutritional requirements. Nevertheless, not only requires adequate education and training of the patient or others involved in handling the catheter, but also is associated with several complications, like liver disease, pancreatitis, glomerulonephritis and catheter-related complications (sepsis and thrombosis). In fact, TPN-related complications are one of the main causes of death of patients with CIPO.
For these reasons we tried to delay the introduction of home TPN for as long as the patient was able to tolerate the enteral nutritional supplements (liquid supplements). During this period of time we had the opportunity to get more informed about home TPN, educate the patient about handling the catheter, about the risks of home TPN and provide adequate social conditions and nurse support at home for this type of nutritional therapy.
Unfortunately, this patients clinical course follows the usual natural history of the disease, with progressive worsening of symptoms, inconsistent response to the various types of therapy available, ultimately evolving to intestinal failure. Studies on the natural history of CIP have found the disorder to be nearly always serious, with a tendency toward progressive worsening of symptoms. Most patients exhibit weight loss and nutritional deficiencies due to limited oral intake and poor absorption associated with small intestine bacterial overgrowth. Approximately 33% of patients end up on home TPN.9,10
References
1. Stanghellini V, Cogliandro RF, De Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440-52. [ Links ]
2. Antonucci A, Fronzoni L, Cogliandro L, Cogliandro RF, Caputo C, De Giorgio R, et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol. 2008;14:2953-61. [ Links ]
3. Connor FL, Di Lorenzo C. Chronic intestinal pseudoobstruction: assessment and management. Gastroenterology. 2006;130:S29-36. [ Links ]
4. Muñoz MT, Solis Herruzo JA. Chronic intestinal pseudoobstruction. Rev Esp Enferm Dig. 2007;99:100-11. [ Links ]
5. De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, et al. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872-83. [ Links ]
6. De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53:1549-52. [ Links ]
7. Smith VV, Gregson N, Foggensteiner L, Neale G, Milla PJ. Acquired intestinal aganglionosis and circulating autoantibodies without neoplasia or other neural envolvement. Gastroenterology. 1997;112:1366-71. [ Links ]
8. De Giorgio R, Barbara G, Stanghellini V, De Ponti F, Salvioli B, Tonini M, et al. Clinical and morphofunctional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: a study of three cases. Am J Gastroenterol. 2002;97:2454-9. [ Links ]
9. Mann Sd, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut. 1997;41:675-81. [ Links ]
10. Stanghellini V, Cogliandro RF, De Giorgio R, Barbara G, Morselli-Labate AM, Cogliandro L, et al. Natural history of chronic idiopathic intestinal pseudo-obstruction in adults: a single center study. Clin Gastroenterol Hepatol. 2005;3:449-58. [ Links ]
*Corresponding author
E-mail address: pimentelrita80@gmail.com (R. Pimentel).
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical disclosures
Protecting people and pets
The authors declare that for this investigation, experimente is not conducted on humans and/or animals.
Confidentiality of data. The authors declare that they have followed the protocols of his work center on the publication of data from patients.
Right to privacy and consent in writing
The authors declare that they have received written consente for patients and/or subjects mentioned in article consent. The corresponding author should be in possession of this document.
Received 25 February 2014; accepted 28 July 2014














