Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.22 no.2 Lisboa abr. 2015
https://doi.org/10.1016/j.jpge.2015.01.009
ORIGINAL ARTICLE
Hepatitis B Virus Inactive Carriers: Which Follow-up Strategy?
Portadores Inactivos do Vírus da Hepatite B: Que Estratégia de Follow-up?
Maria João Magalhães∗, Isabel Pedroto
Department of Gastroenterology, Hospital de Santo António, Centro Hospitalar do Porto, Porto, Portugal
* Corresponding author.
ABSTRACT
Introduction: The natural history of patients with inactive hepatitis B virus (HBV) is still unclear, persisting doubts about the optimal management of these patients.
Aim: To evaluate the long-term outcome in a cohort of hepatitis B inactive carriers.
Methods: We conducted a retrospective study in a cohort of 100 HBV inactive carriers (categorized after quarterly determinations of serum ALT and HBV DNA over one year) and analyzed the results of serial determinations of HBV DNA and alanine transaminase (ALT). The HBV DNA was quantified by Cobas TaqMan®. We used the Spearmans rank correlation coefficient to evaluate the correlation between the serum ALT and HBV DNA.
Results: We studied 100 HBV inactive carriers (53% females, mean age 48.7±13.8 years, range 16-77 year). Vertical transmission was identified in 18%. The mean follow-up time was 4.6±2.5 (2-13) years. Two patients had transient elevation of ALT (alcohol and drugs). We observed clearance of hepatitis B surface antigen (HBsAg) in four patients (4%) and biological and virological reactivation in 10% (from the 4th year of follow-up). Mild lesions were found in the 12 patients in whom liver biopsy was performed; genotypes A and D predominated. Viral load and serum ALT levels were unremarkable in 90% of the patients. There was no significant correlation (p > 0.05) between the values of ALT and HBV DNA throughout the follow-up.
Conclusion: The management strategy, using both patterns of biochemical and virologic activity, seems adequate. The lack of correlation between the values of ALT and HBV DNA caveat its effectiveness and the stability of the levels of HBV DNA and ALT in most patients suggests that the prognosis of the inactive carriers, when defined accurately, is mostly benign. Further studies, including ones with new tests available, are needed to standardize and improve e the management of this group of patients.
Keywords: Carrier State; Hepatitis B, Chronic/virology; Hepatitis B virus
RESUMO
Introdução: A história natural dos portadores inactivos do vírus da hepatite B (VHB) é ainda pouco clara, persistindo dúvidas acerca da melhor estratégia de seguimento.
Objectivo: Avaliar o outcome num coorte de portadores inactivos VHB.
Métodos: Estudo retrospectivo num coorte de 100 portadores inactivos do VHB (categorizados após determinações seriadas trimestrais da ALT sérica e do ADN VHB durante 1 ano) através de doseamentos seriados anuais do ADN VHB e da ALT. O ADN do VHB foi quantificado através do Cobas TaqMan®. Utilizou-se o coeficiente de correlação estatística não paramétrico de Spearman para avaliar a existência de correlação entre os valores séricos da AL T e do ADN VHB.
Resultados: Dos 100 doentes incluídos, 53% são do sexo feminino; a idade média é de 48,7±13,8 (16-77) anos. A transmissão vertical foi identificada em 18%. A duração média do follow-up é de 4,6±2,5 (2-13) anos. Dois doentes apresentaram elevação transitória da ALT (álcool e fármacos). Observou-se clearance do AgHBs em 4 doentes (4%) e reativação biológica e virológica em 10% (a partir do 4◦ ano de follow-up). Foi realizada biopsia hepática em 12 doentes que apresentavam lesões mínimas. Os genótipos A e D foram os mais comuns. A carga viral e os níveis séricos da ALT mantiveram-se estáveis na maioria dos doentes (90%). Não foi observada correlação significativa (p>0,05) entre os valores da ALT e do ADN do VHB, ao longo do follow-up.
Conclusões: A estabilidade dos níveis do ADN do VHB e da ALT na maioria dos doentes e a ausência de correlação entre os valores da ALT e do ADN do VHB ressalvam a eficácia desta estratégia de follow-up e sugerem que o prognóstico dos portadores inactivos, quando definidos com rigor, é sobretudo benigno. São necessários mais estudos, com novos e promissores métodos, para optimizar e uniformizar a abordagem deste grupo de doentes.
Palavras-Chave: Hepatite B Crónica/virologia; Portador Inactivo; Vírus da Hepatite B
1. Introduction
Infection with hepatitis B virus (HBV) is a major public health problem, affecting about 350-400 million people worldwide. It is endemic in Asia, sub-Saharan Africa, the South Pacific Region, Australia, New Zealand and in some populations of South America and the Middle East.1,2
Perinatal infection or horizontal infection early in childhood are the main routes of HBV transmission in high endemic areas, whereas in low endemic regions, such as western countries, hepatitis B is primarily a disease of adolescents and adults as a result of high risk sexual activity and injection drug use.3 The probability of becoming chronically infected is higher in individuals infected perinatally (90%) or during childhood (20-30%) when considering standing before an immature immune system comparing to immunocompetent individuals infected in adulthood (<1%).1,3 Between 15 and 40% of chronically infected individuals may develop severe liver disease and hepatocellular carcinoma (HCC), while the remaining become inactive carriers.1
The natural history of chronic HBV infection can be divided into five phases, not necessarily sequential, that depend on the virus-host interaction: (i) Immune-tolerant phase: This phase may persist for 10-30 years in individuals infected perinatally or in the first years of life. (ii) Immune-reactive phase (HBeAg-positive): This phase is more frequent and/or more easily achieved in individuals infected in adulthood. (iii) State of inactive HBV carrier: It may occur after HBeAg seroconversion to anti-HBe. It is characterized by the absence of HBeAg and presence of anti-HBe, serum HBV DNA levels <2000 IU/mL, normal ALT levels and histologically minimal or no necroinflammation, slight fibrosis or even normal liver. (iv) HBeAg-negative chronic hepatitis B (v) HBsAg-negative phase: In these patients immunosuppression, such as chemotherapy or after transplantation, can lead to reactivation of hepatitis B, hence antiviral treatment is indicated.4
HBV Inactive carriers, previously designated as healthy carriers, asymptomatic carriers of HBsAg, constitute the largest group of patients chronically infected with HBV. It is estimated that about 300 million individuals are in this phase. More recently it has been proposed the term inactive carrier state due to the possibility of reversing this condition.
The prognosis of the inactive carriers is usually benign. Long-term follow-up studies (up to 18 years) show that the vast majority has a sustained biochemical remission and very low risk of progression to cirrhosis or hepatocellular carcinoma (HCC).2 Rarely, some patients, even without cirrhosis, can develop HCC during the state of inactive carrier and, in addition, from 10 to 34% of these patients may have a spontaneous reactivation during follow-up, with or without sero-reversion to HBeAg. The risk of reactivation is higher in the following years after HBeAg seroconversion and decreases with time, although it can be present after years of inactivity. The occurrence of multiple episodes of reactivation or sustained reactivation can cause progressive liver damage and even hepatic decompensation.5,6 Acute flares of hepatitis occurring during the inactive HBsAg carrier state should be distinguished from super-infection with other hepatotropic viruses that may occur in as many as 20-30% and can be associated with an increased risk of fulminant hepatic failure.2,7 Treatment is not recommended in this group of patients,8,9 neither routine liver biopsy, as it was considered that potential for histological lesions is minimal. For the follow-up of this particular group of individuals with chronic HBV infection periodic monitoring of ALT (every 3, 6 or 12 months) to detect reactivation is recommended. Regarding HBV DNA, recent data show the need to complement the value of ALT with HBV DNA to avoid misclassification of the infection profile, taking into account the fluctuations of HBV DNA.
It was recently proposed the use of HBsAg quantification for categorization of this group. A study by Brunetto et al.10 says that the serum HBV DNA and HBsAg levels provide complementary information to distinguish HBV carriers of inactive subjects in genotype D. The combination of quantification of HBV DNA (<2000 IU/mL) and HBsAg (<1000 IU/mL) allows the identification of inactive carriers with a high diagnostic accuracy (94.3%), which is comparable with annual monitoring of HBV DNA and ALT. These data require further analysis.
HBsAg status should be monitored to detect a spontaneous seroconversion, which is estimated to occur at about 0.5-1% per year and increases with age. Of these, just over half acquire the anti-HBs antibody, suggesting that in these cases the HBV infection has not been eliminated, but the production of HBsAg decreased to a value lower than the sensitivity of detection methods.
Inactive carriers have a very low risk of developing cirrhosis, below 0.1 per 100 patient-years.11 The inactive carriers with cirrhosis account for 1% of the total. In these cases, treatment is recommended to reduce this risk of disease progression. The screening for cirrhosis in inactive carriers must be systematic, being up to now based only on clinical, analytical and imaging findings.9 Non-invasive methods to assess the presence of fibrosis, such as elastography and serum tests, may prove to be a more effective way to detect suspected cases of cirrhosis.12
Although the risk of cancer is increased regarding the population not exposed to HBV, the overall risk is low (0.06% per year) hence systematic screening of HCC does not seem to be justified.6,13 Regardless of the severity of the underlying liver disease, the risk of HCC is negligible in Caucasians inactive carriers, but increases about 10 times in Asian inactive carriers.13
2. Objective
The aim of this study was to evaluate the long-term outcome of a cohort of HBV inactive carriers, through biochemical and virological monitoring
3. Patients and methods
3.1. Patients
A retrospective analysis of outpatients of the Hepatology consultation between 1997 and 2010 was performed. One hundred patients were identified, with criteria to be classified as inactive carriers (after quarterly determinations of ALT and DNA HBV during one year). Patients with the following characteristics were included: presence of serum HBsAg and anti-HBe, without HBeAg; HBV DNA levels <2000 IU/mL; repeatedly normal ALT levels. Other inclusion criteria were absence of evidence of infection with hepatitis C virus (undetectable anti-HCV), no antibodies of human immunodeficiency virus, no anti-d antibodies and exclusion of other causes of chronic liver disease (alcohol consumption, virus, NAFLD, hepatotoxic drugs, chronic autoimmune hepatitis, hemochromatosis, Wilsons disease, and a1-antitrypsin deficiency).
3.2. Methods
The virological markers, including HBsAg, HBeAg, anti-HBc, anti-HBe and anti-HBs were determined with standard procedures. Serum ALT assays levels were assessed by Hitachi® robot 911, using Boehringer-Mannheim® reagents. The upper limit of 30 IU/mL for men and 19 IU/mL for women was used.14 The HBV DNA was quantified by Amplicor HBV Monitor® Kit (Roche) and COBAS® AmpliPrep/COBAS® TaqMan® HBV test (Roche).
Percutaneous liver biopsy was performed in 12 patients. Liver histology was classified according to the score system defined by Ishak et al.15 that includes a fibrosis score and a score for necroinflammation.
Statistical analysis was performed with SPSS software® (v.11, 5, SPSS Inc., Chicago, IL). For quantitative variables (ALT and HBV DNA), the coefficient of nonparametric Spearman correlation was used.
4. Results
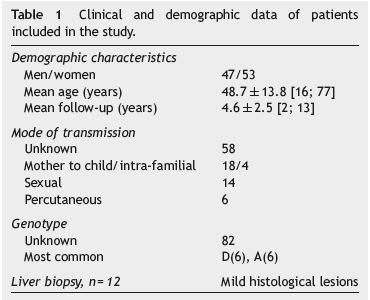
One hundred HBV inactive carriers were studied, of whom 53% were female, mean age 48.7±13.8 (16-77) years. The mean follow-up period was 4.6±2.5 (2-13) years. Vertical transmission was identified in 18% of patients. Genotypes A and D were the most common. The characteristics of this group of patients are reported in Table 1.
During follow-up, there was an increase in viremia in a total of 33 patients, but in 23 of those HBV DNA levels remained below 2000 IU/mL.
There was biological and virological reactivation in 10% (4 men, 6 women) on the 4th year of follow-up. Of these, all men had an ALT value greater than 30 IU/L, at inclusion, and three of the six women had a value greater than 19 IU/L and the HBV DNA was below the limit of quantification in all of them. No correlation between the values of ALT and HBV DNA (p > 0.05) was observed. The increased viremia preceded the elevation of ALT levels in six of ten cases.
Clearance of HBsAg was documented in four patients (4%) (2 men, 2 women) who showed persistently normal ALT values and HBV DNA <20 IU/mL, except in one case that showed a transient viremia rise in the year prior to clearance, although with a value lower than 2000 IU/mL. We were unable to determine the time of infection in this group.
Three of the patients became anti-HBs positive. Two male patients presented transient elevation of ALT, one of them in the context of alcohol consumption and the other because of drug hepatotoxicity (amoxicillin and clavulanic acid).
In the 12 patients in whom liver biopsy was performed, histologic activity was mild, without fibrosis (Ishak <6).
There was no case of liver disease progression, particularly with cirrhogenic evolution or diagnosis of HCC.
5. Discussion
This study was performed in a homogeneous and large cohort of HBV inactive carriers. The correct identification of the inactive carriers has important prognostic implications, since these patients survival is comparable to that of non-infected population, at least in Western studies. According to international guidelines, a correct approach to chronic HBV infection requires an accurate differential diagnosis between chronic hepatitis and the inactive carriers through the serial (quarterly) determination of ALT levels and HBV DNA for at least one year. Some data suggest that the use of HBV DNA and ALT alone to define an inactive carrier, without resort to liver biopsy, may not detect significant histological disease in about 10% of patients.16 Based on this, liver biopsy was performed in only 12 patients. We acknowledge this as limitation, as it is a retrospective study, although the results are similar to other studies that consider there is minimal or no necroinflammatory activity in the inactive carrier state. Since liver biopsy is seldom recommended in these patients, there are few confirmations of these findings and liver histologic changes in this group are difficult to assess. As liver biopsy is an invasive method of evaluating liver fibrosis, and carries small risk of complications, we consider appropriate to not routinely perform biopsy in these asymptomatic group of patients.17 Despite some limitations, transient elastography appears an ideal approach for identifying liver fibrosis in all inactive carriers.17 Recent studies on HBsAg level in different population of chronic HBV infection have shown that HBsAg level is lowest in the inactive carrier patient and HbsAg quantitation may help to differentiate between active and inactive HBeAg-negative HBsAg carrier state. The combination of serum HBsAg and HBV DNA levels shows promising results as a single-point measurement, instead of quarterly laboratorial monitoring over a year, for identifying inactive carriers.10,13,16-20
The results show that in 90% of patients most (77%) had no detectable serum HBV DNA and 23% had levels below 2000 IU/mL.
In the Western countries, the reactivation is rare and when it occurs is due mainly to the presence of cofactors such as alcohol or drugs. Significant predictors of mortality in HBsAg-positive patients are the presence of medical comorbidities, older age at diagnosis, and abnormal GGT levels.1,21-23 Another study concluded that the majority of inactive carriers have a low level of viral replication.24
HBV reactivation occurred in ten of our patients, which is in line with published data that estimate that reactivation occurs in 10-34% of these patients.25,26
Spontaneous loss of HBsAg is about 1-3% per year and HCC is rare.9,16 There was clearance of HBsAg in 4% of patients and seroconversion occurred with the appearance of anti-HBs in three patients. In these patients, the persistently normal values of ALT and HBV DNA <2000 IU/mL suggest that the loss of HBsAg may occur below the threshold of HBV replication, but it does not mean virus eradication. It was reported that HBV DNA can still be detected with PCR in patients with chronic hepatitis B who develop HBsAg seroconversion either spontaneously or after therapy, although a longer follow-up is necessary to confirm this result.24,27
The results of the present study show that serial monitoring with persistently normal ALT values and HBV DNA level <2000 IU/mL in HBeAg-negative HBsAg seropositive persons are appropriate criteria to define HBV inactive carriers. The follow-up and outcome suggest that the prognosis of HBV inactive carriers, when accurately defined, is benign.
6. Conclusion
The natural history of the HBV inactive carriers is not totally clear and there are few studies about this group. The prognosis is generally good, but the risk of reactivation, and consequent liver disease complications namely HCC, remains present even after several years of inactivity.28 Thus, a surveillance strategy is needed. With this study, using the recommended strategy of regularly monitor ALT and viremia, we found similar results to previously published data.
It is our believe that more studies with large cohorts are needed to define the most adequate management strategy to these patients, including data about the promising AgHBs quantification and transient elastography.
References
1. Manno M, Cammá C, Schepis F, Bassi F, Gelmini R, Giannini F, et al. Natural history of chronic HBV carriers in Northern Italy: morbidity and mortality after 30 years. Gastroenterology. 2004;127:756-63. [ Links ]
2. Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39:S50-8. [ Links ]
3. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-52. [ Links ]
4. Lok ASF, McMahon BJ, Chronic Hepatitis B. Hepatology. 2007;45:507-39. [ Links ]
5. Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J. 2005;2:82. [ Links ]
6. Andreani T. HBV-carriers: when is monitoring and surveillance sufficient? Clin Res Hepatol Gastroenterol. 2011;35:813-8. [ Links ]
7. Perillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1099-122. [ Links ]
8. Lok AS, McMahon BJ. Chronic hepatic tis B: update. Hepatology. 2009;50:661-2. [ Links ]
9. EASL Clinical Practice Guidelines. Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-85. [ Links ]
10. Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483-90. [ Links ]
11. Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAG seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-7. [ Links ]
12. Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-50. [ Links ]
13. Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver related death. Gastroenterology. 2010;138:1747-54. [ Links ]
14. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [ Links ]
15. Ishak K, Baptista A, Bianchi L, Callea F, DeGroote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-9. [ Links ]
16. Villa E, Fattovich G, Mauro A, Pasino M. Natural history of chronic HBV infection: special emphasis on the prognostic implications of the inactive carrier state versus chronic hepatitis. Dig Liver Dis. 2011;43:S8-14. [ Links ]
17. Pita I, Horta-Vale A, Cardoso H, Macedo G. Hepatitis B inactive carriers: an overlooked population? GE Port J Gastroenterol. 2014;21:241-9. [ Links ]
18. Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508-13. [ Links ]
19. Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. Hepatitis B surface antigen (HBsAG) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-22. [ Links ]
20. Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52:1232-41. [ Links ]
21. Kato Y, Nakao K, Hamasaki K, Nakata K, Kusumoto Y, Eguchi K. Spontaneous loss of hepatitis B surface antigen in chronic carriers, based on along-term follow-up study in Goto island, Japan. J Gastroenterol. 2000;35:201-5. [ Links ]
22. Hassan MM, Hwang L-Y, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-13. [ Links ]
23. Oshima A, Tsukuma H, Hiyama T, Fujimoto I, Yamano H, Tanaka M. Follow-up study of HBsAg-positive blood donors with special reference to effect of drinking and smoking on development of liver cancer. Int J Cancer. 1984;34:1389-96. [ Links ]
24. Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Olivier S, et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol. 2002;36:543-6. [ Links ]
25. Davis GL, Hoofnagle JH,Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984;86:230-5. [ Links ]
26. Fattovich G, Brollo L, Alberti A, Realdi G, Pontisso P, Giustina G, et al. Spontaneous reactivation of hepatitis B virus infection in patients with chronic type B hepatitis. Liver. 1990;10:141-6. [ Links ]
27. Loriot MA, Marcellin P, Bismuth E, Martinot-Peignoux M, Boyer N, Degott C, et al. Demonstration of hepatitis B virus DNA by polymerase chain reaction in the serum and the liver after spontaneous or therapeutically induced HBeAg to anti-HBe or HBsAg to anti-HBs seroconversion in patients with chronic hepatitis B. Hepatology. 1992;15:32-6. [ Links ]
28. Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-7. [ Links ]
* Corresponding author
E-mail address: mj.magalhaes@gmail.com (M.J. Magalhães).
Ethical responsibility
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Received 23 November 2014; accepted 20 January 2015














