Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.22 no.4 Lisboa ago. 2015
https://doi.org/10.1016/j.jpge.2015.03.008
ENDOSCOPIC SNAPSHOT
Duodenal Variceal Bleeding Successfully Treated by Endoscopic N-butyl-2-cyanoacrylate Injection
Tratamento Bem-sucedido de Hemorragia Variceal Duodenal por Injecção Endoscópica de N-butil-2-cianoacrilato
Mariana Costa∗, Gonçalo Ramos
Gastroenterology Department, Centro Hospitalar de Lisboa Central, Lisbon, Portugal
* Corresponding author.
Keywords: Duodenum; Enbucrilate; Gastrointestinal Hemorrhage; Hemostasis, Endoscopic
Palavras-chave: Duodeno; Embucrilato; Hemorragia Gastrointestinal; Hemostase Endoscópica
Gastroesophageal variceal bleeding is the most common cause of upper gastrointestinal bleeding in patients with liver cirrhosis. In contrast, duodenal varices are rare and their potential to bleed is low. However, when it occurs, it can be fatal - mortality rate of 35-40%.1 Although the recognition of ectopic varices has long been described, therapeutic procedures have not yet been established for bleeding from duodenal varices. Endoscopic band ligation, sclerotherapy, transjugular intrahepatic portosystemic shunt, balloon-occluded retrograde transvenous obliteration, and resection of a segment of bleeding site have been used for treatment.2-5
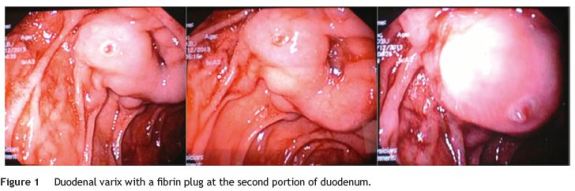
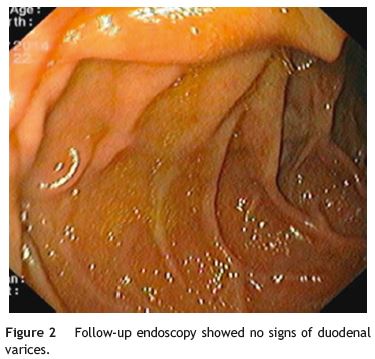
We present a case of a 48-year-old man with a known history of liver cirrhosis secondary to chronic hepatitis C infection. He was admitted in emergency department due to hematemesis and melena for 24 h. He was hemodynamically stable, and had a hemoglobin level of 7.1 g/dL. The patient was treated with intravenous (IV) pantoprazole, IV octreotide and IV cetfriaxone. Emergency esophagogastroduodenoscopy revealed small esophageal varices without stigmata of hemorrhage. Apart from mild portal gastropathy, no other gastric lesions suggestive of bleeding were noted. Finally, a duodenal varix with a fibrin plug at the second portion, approximately 1 cm in diameter, was found (Fig. 1). N-butyl-2-cyanoacrylate (histoacryl) was injected to the duodenal varix, and bleeding was successfully controlled. Subsequently, the patient remained hemodynamically stable, with no recurrent bleeding. Six months later, a follow-up endoscopy showed large esophageal varices, which were treated with endoscopic band ligation, and severe hypertensive gastropathy. There were not any signs of duodenal varices (Fig. 2).
References
1. Khouqeer F, Morrow C, Jordan P. Duodenal varices as a cause of massive upper gastrointestinal bleeding. Surgery. 1987;102:548-52. [ Links ]
2. Matsui S, Kudo M, Ichikawa T, Okada M, Miyabe Y. The clinical characteristics, endoscopic treatment, and prognosis for patients presenting with duodenal varices. Hepatogastroenterology. 2008;55:959-62. [ Links ]
3. Tan NC, Ibrahim S, Tay KH. Successful management of a bleeding duodenal varix by endoscopic banding. Singap Med J. 2005;46:723-5. [ Links ]
4. Seo YS, Kwon YD, Park S, Keum B, Park BJ, KimYS, et al. Complete eradication of duodenal varices after endoscopic injection sclerotherapy with ethanolamine oleate: a case report. Gastrointest Endosc. 2008;67:759-62. [ Links ]
5. Kochar N, Tripathi D, McAvoy NC, Ireland H, Redhead DN, Hayes PC. Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther. 2008;28:294-303. [ Links ]
* Corresponding author.
E-mail address: mariananunocosta@gmail.com (M. Costa).
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Received 29 March 2015; accepted 31 March 2015














