Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.23 no.5 Lisboa out. 2016
https://doi.org/10.1016/j.jpge.2016.01.006
CLINICAL CASE
Cystic Pancreatic Lymphangioma – Diagnostic Role of Endoscopic Ultrasound
Linfangiomas Quísticos Pancreáticos – A Importância da Ecoendoscopia no seu Diagnóstico
Diana Carvalho a, *, Mariana Costa a, Pedro Russo a, Luís Simas a, Teresa Baptista b, Gonçalo Ramos a
a Gastroenterology and Hepatology Department, Hospital Santo António dos Capuchos, Centro Hospitalar de Lisboa Central, Lisbon, Portugal
b Anesthesiology Department, Hospital Santo António dos Capuchos, Centro Hospitalar de Lisboa Central, Lisbon, Portugal
* Corresponding author.
ABSTRACT
Pancreatic cystic lymphangiomas are rare benign lesions that arise from lymphatic vessels, accounting for less than 0.2% of all pancreatic cysts. Typically it is asymptomatic and discovery occurs during imaging exams for non-pancreatic disease. In the past, a definite diagnosis was made through surgery, with complete resection of all tumoral tissue to prevent recurrence. Nowadays, the development of endoscopic ultrasound (EUS) made it possible to identify these cysts combining morphologic ultrasound features, macroscopic aspirated fluid appearance, biochemical and cytological evaluation of the sample. We report two cases of cystic pancreatic lymphangioma diagnosed through EUS, allowing conservative management without surgery. These cases show that cystic pancreatic lymphangioma should be considered in the differential diagnosis of cystic pancreatic lesions and that EUS is an important tool for their recognition.
Keywords: Lymphangioma, Cystic. Pancreatic Neoplasms. Endosonography.
RESUMO
Os linfangiomas quísticos pancreáticos são lesões benignas raras com origem em vasos linfáticos, correspondendo a menos de 0,2% da totalidade de quistos pancreáticos. Na maioria são assintomáticos sendo a sua descoberta incidental. Tradicionalmente o seu diagnóstico era cirúrgico, com completa ressecção de todo o tecido tumoral para prevenir recorrência. Actualmente, o desenvolvimento da ecoendoscopia (EUS) permitiu identificar estes quistos combinando as suas características ultrasonográficas, aparência macroscópica do fluido aspirado, e avaliação bioquímica e citológica da amostra. Os autores descrevem dois casos de linfangiomas quísticos pancreáticos diagnosticados por EUS, permitindo uma abordagem conservadora. Estes demonstram que os linfangiomas quísticos pancreáticos devem ser considerados no diagnóstico diferencial de lesões quísticas pancreáticas e que a EUS é importante no seu reconhecimento.
Palavras-chave: Linfangioma Quístico. Neoplasias do Pancreas. Ecoendoscopia.
1. Introduction
Lymphangiomas are benign tumors that result from the blockage of lymphatic flow leading to the development of lymphangiectasias.1 This phenomenon could be related to congenital malformations or obstructions secondary to inflammatory process (for example: infections), abdominal trauma, surgery or radiotherapy.2 Pancreatic lymphangioma are very rare, representing less than 1% of all lymphangiomas and 0.2% of pancreatic lesions.3 In literature few cases and small series are described, however the majority are diagnosed after surgery. With the advent of endoscopic ultrasound (EUS) it became possible to establish a definite preoperative diagnosis through the combination of its morphologic features on ultrasonography, and the analysis of cyst fluid by fine-needle aspiration (FNA), namely its biochemical and cytological characteristics.4,5
We present two cases of pancreatic lymphangioma discovered incidentally, in which the definitive diagnosis was made by EUS with FNA, allowing a conservative approach without need for surgery.
2. Case 1
A 75 year-old asymptomatic male was referred for a pancreatic cyst incidentally detected in computerized tomography (CT). The lesion was identified in the pancreatic uncinate process with 54 mm × 37 mm, mostly hypodense but with a small area with contrast enhancement. There was no personal or family history of pancreatic diseases.
A magnetic resonance imaging (MRI) was performed, revealing in the same location and in contact with the inferior vena cava, a grossly rounded lesion with 52 mm × 47 mm, hypointense on T1 and hyperintense on T2, containing septa and very slight contrast uptake. These aspects were suggestive of serous or mucinous cystadenoma.
To obtain a definitive diagnosis the patient was submitted to EUS evaluation with a linear echoendoscope. EUS showed a cystic lesion reaching 44.5 mm of diameter in the uncinate process of the pancreas, with thin septa converging to the center. There was no wall thickness or parietal nodules (Fig. 1). FNA was performed using 22 gauge EUS-FNA needle in a single function, resulting in aspiration of a milky-white fluid; string sign negative (Fig. 2).
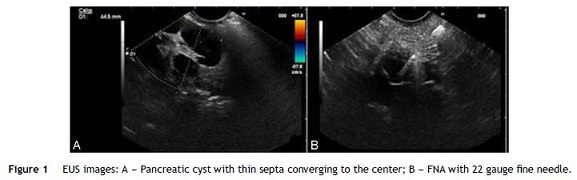
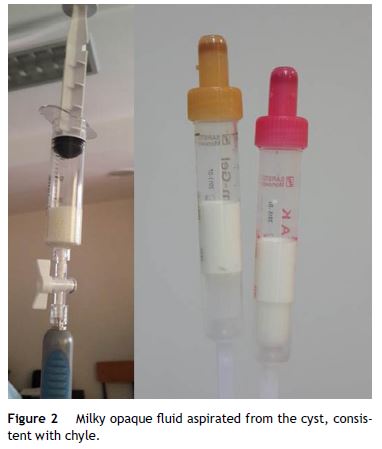
The remaining pancreas seemed to be normal at EUS.
Prophylactically endovenous ciprofloxacin was administrated before FNA in a single dose, and then orally for the next 4 days after the function.
The biochemical examination of the fluid revealed an elevated triglyceride level of 12,306 mg/dL, with amylase 90 U/L and carcinoembryonic antigen (CEA) 10.4 ng/mL. The cytological evaluation was inconclusive, with a very small amount of cells in the sample.
The EUS findings along with the fluid's macroscopic appearance and triglyceride levels were diagnostic of pancreatic lymphangioma. Since the patient was asymptomatic and taking in account the risks of an extended surgery, it was decided by multidisciplinary team to keep following the patient with imaging studies.
3. Case 2
A 54 year-old woman presented with chronic middle upper abdominal pain in 2014. She had a medical history of breast cancer in 2010, treated with mastectomy, chemotherapy and radiotherapy. The physical examination and laboratory studies were normal. Abdominal CT demonstrated a large cystic lesion (136 mm × 57 mm × 103 mm) involving all the pancreatic segments. The main arterial (celiac trunk, hepatic, splenic and, in part, superior mesenteric artery) and venous (portal confluent and vein) vessels were wrapped within the cyst, without vascular wall invasion (Fig. 3).
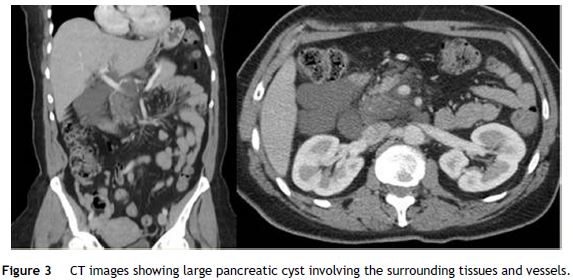
EUS was performed revealing a multiseptated voluminous cystic lesion occupying the entire pancreatic area, with impure anechoid content. The largest locules were located in the cephalic region and the distal segments showed a honeycomb microcystic pattern (Fig. 4). Given the size of the lesion, it was not possible to document relationship with the pancreatic duct. Celiac trunk and superior mesenteric artery seemed to be permeable in their origin, but they were involved by the cyst in their distal segments. Portal vein was also wrapped by the lesion. FNA was performed using a 19 gauge EUS-FNA needle in a single function. The fluid was yellowish, with chylous appearance, string sign negative (Fig. 5).
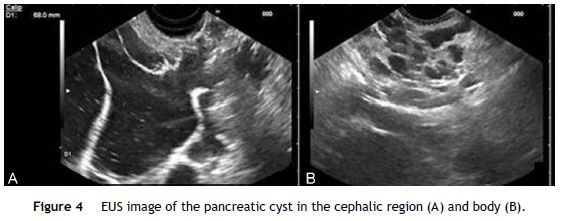
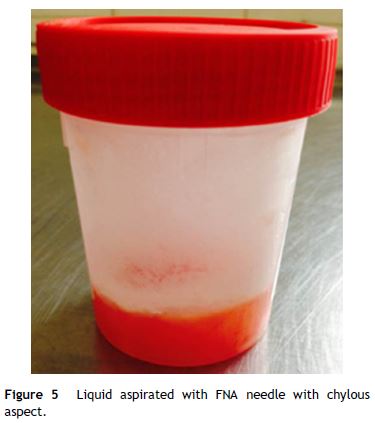
Biochemical analysis revealed a lipemic content with low amylase (42 U/L) and CEA (0.7 ng/mL) levels. The cytological exam showed a large population of small mature lymphocytes.
The diagnosis of pancreatic lymphangioma was made based on the morphologic findings, chylous macroscopic nature of the fluid and cytochemical features. After multidisciplinary discussion, the patient was proposed for conservative management.
4. Discussion
Pancreatic cystic lymphangioma is a rare benign type of cystic pancreatic lesions, accounting for less than 100 cases described since its first report by Koch in 1913.6 They are more frequently found in females (ratio 2:1), affecting all age groups with the same incidence. The majority are localized in pancreatic body or tail, although they can arise from other parts of the pancreas.7 Tumor size is variable, with diameter ranging from 3 to 20 cm (average 12 cm).8
Usually it is asymptomatic, being discovered incidentally in radiological exams.3 Depending on their size, they can become symptomatic: the most common complaints are abdominal pain and palpable abdominal mass, followed by nausea, and vomiting.9 Although rarely, it can manifest as acute abdomen due to complications such as cyst rupture, volvulus, infection or intracystic hemorrhage.8 Weight loss, fever or pancreatitis are not usually associated with these lesions.3
Traditionally, an accurate diagnosis before surgery was difficult since imaging studies were not sufficient to characterize the lesion.10 Its differential diagnosis includes pancreatic pseudocysts, serous and mucinous cystadenomas, intraductal papillary mucinous neoplasm, congenital neoplasms, pancreatic ductal carcinoma with cystic degeneration and echinococcal cysts.11
There are not any specific or significant laboratory abnormalities that confirm the diagnosis of pancreatic lymphangioma.12 Imaging exams such as plain abdomen X-rays, ultrasonography (US), CT or MRI can be helpful in the workup, however they cannot establish a definite diagnosis of lymphangioma to detriment of the other diseases previously cited.2,13
In large tumors, plain abdominal X-rays can identify signs of bowel dislocation or blockage.13 US typically shows a polycystic tumor, rarely with calcifications.11 CT and MRI are useful to determine the size and location of the cyst, its relation with surrounding structures and organs, and to plan the surgical approach if necessary.2,13 In these exams they appear as a well-circumscribed, encapsulated, water-isodense, uni or multilocular lesions with thin septa.11,14
EUS is the optimal exam to reveal internal details of pancreatic lesions, with the advantage of allowing FNA of the cyst fluid for diagnostic purpose. The pancreatic lymphangiomas have a variable appearance on EUS. They can be uni or multilocular, and it is possible to find both micro and macro-cysts in the same lesion. Usually the contents are anechoic, with thin and delicate septae, without debris or solid components.10 It is important to note that these ultrasonographic features are not exclusive of lymphangiomas, being present in other pancreatic lesions including some of the more concerning, namely mucinous lesions.3
A chylous aspirated fluid, with a milky-white appearance and an elevated triglyceride level its diagnostic of pancreatic lymphangioma.3,11 The levels of amylase and CEA are low in these cysts.5
The diagnosis of lymphangioma can be pathologically confirmed, where they emerge as a lesion made of dilated cystic spaces lined by endothelial cells and containing proteinaceous eosinophilic fluid. These spaces are separated by thin septa composed of smooth muscle cells, mature lymphocytes and some histiocytes.14 The cytological exam of the aspirated fluid shows a large population of small mature lymphocytes.5
Immunohistochemical reactivity is positive for CD 31, CD 34 and factor VIII-R, which are markers for lymphatic and capillary endothelial cells.11
Considering that lymphangiomas are benign lesions, a conservative approach with close follow-up is reasonable if definitive diagnosis is made by EUS. However, they can be locally invasive and the potential for complications (for example: hemorrhage, obstruction, rupture, torsion or infection) remains. This should be balanced with the risks of a complete excision of the lesion,3 which could involve a tumorectomy,14 a Whipple procedure or a distal pancreatectomy.11 For curative intent it is important to remove all tumoral tissue to prevent recurrence.9
In conclusion, lymphangiomas should be considered in the differential diagnosis of pancreatic cysts. They can be accurately diagnosed by EUS with FNA, allowing conservative management in selected patients.
References
1. Colovic RB, Grubor NM, Micev MT, Atkinson HD, Rankovic VI, Jagodic MM. Cystic lymphangioma of the pancreas. World J Gastroenterol. 2008;14:6873-5. [ Links ]
2. Sohn BK, Cho CH, Chae HD. Cystic lymphangioma of the pancreas. J Korean Surg Soc. 2011;81:141-5. [ Links ]
3. Coe AW, Evans J, Conway J. Pancreas cystic lymphangioma diagnosed with EUS-FNA. J Pancreas. 2012;13:282-2848. [ Links ]
4. Applebaum B, Cunningham JT. Two cases of cystic lymphangioma of the pancreas. Endoscopy. 2006;38:E24-5. [ Links ]
5. Fonseca R, Pitman MB. Lymphangioma of the pancreas: a multimodal approach to pre-operative diagnosis. Cytopathology. 2013;24:172-6. [ Links ]
6. Cicy PJ, Sansho EU, Lekshmidevi P, Umman P, Varghese S, Kurian JS. Giant cystic lymphangioma of pancreas – a rarity. World J Pathol. 2014;4:72-6.
7. Mortelé KJ. Cystic pancreatic neoplasms: imaging features and management strategy. Semin Roentgenol. 2013;48:253-63. [ Links ]
8. Gures N, Gurluler E, Alim A, Berber I, Gurkan A. Cystic pancreatic lymphangioma. Rare Tumors. 2012;4:e27. [ Links ]
9. Bona ED, Beltrame V, Blandamura S, Liessi F, Sperti C. Huge cystic lymphangioma of the pancreas mimicking pancreatic cystic neoplasm. Case Rep Med. 2012;2012:951358. [ Links ]
10. Bhatia V, Rastogi A, Saluja SS, Kumar M, Bihari C, Kalayarasan R, et al. Cystic pancreatic lymphangioma. The first report of a preoperative pathological diagnosis by endoscopic ultrasound-guided cyst aspiration. J Pancreas. 2011;12:473-6. [ Links ]
11. Ghatak S, Ray S, Sanyal S, Sonar P.K, Khamrui S, Basu K, et al. An usual case of acute abdomen in adults: giant cystic lymphangioma of the pancreatic head. A clinical case and report of the literature. J Pancreas. 2011;12:266-70. [ Links ]
12. Leung TK, Lee CM, Shen LK, Chen YY. Differential diagnosis of cystic lymphangioma of the pancreas based on imaging features. J Formos Med Assoc. 2006;105:512-7. [ Links ]
13. Mousavi SR, Moradi A, Sobhiyeh MR, Jabbehdari S, Azimi B, Lotfollahzadeh S, et al. A patient with cystic lymphangioma in pancreas. Gastroenterol Hepatol Bed Bench. 2013;6:159-64. [ Links ]
14. Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms of the pancreas revisited. Part IV: Rare cystic neoplasms. Surg Oncol. 2012;21:153-63. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
* Corresponding author.
E-mail address: dianafbcarvalho@gmail.com (D. Carvalho).
Received 4 November, 2015; accepted 10 January 2016














