Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.1 Lisboa fev. 2017
https://doi.org/10.1159/000450870
REVIEW ARTICLE
Evaluation and Management of Gastric Superficial Neoplastic Lesions
Avaliação e Tratamento de Lesões Superficiais Neoplásicas do Estômago
Pedro Pimentel-Nunesa–c, Diogo Libânioa, c, Mário Dinis-Ribeiroa, c
a Department of Gastroenterology, Portuguese Oncology Institute, b Department of Physiology, Faculty of Medicine, University of Porto and c CINTESIS/Department of Biostatistics and Medical Informatics, Faculty of Medicine, University of Porto, Porto, Portugal
* Corresponding author.
ABSTRACT
Gastric cancer is one of the most common and lethal cancers in the world. In Portugal, it is a major health problem presenting one of the highest incidence rates among European countries. In most Western countries, gastric cancer is generally diagnosed in advanced stages. Nevertheless, with the widespread use of upper endoscopy, gastric superficial neoplastic lesions are being increasingly recognized and diagnosed. However, there are no clear recommendations regarding who should be screened for its presence and only recently guidelines concerning the evaluation and management of these lesions were published. In this review, we summarize the current scientific evidence regarding diagnosis and management of gastric superficial neoplastic lesions. Topics like screening, diagnosis, endoscopic evaluation, management, treatment, pathologic evaluation and follow-up of patients with these lesions are covered and areas of future research are discussed. Whenever possible, evidence-based recommendations are made, and on the other cases expert opinion is presented.
Keywords: Gastric neoplasms (MeSH); Endoscopic mucosal resection; Endoscopic submucosal dissection; Early gastric cancer; Gastric superficial neoplasia
RESUMO
O cancro gástrico é um dos tumores mais comum e letal em todo o mundo. Em Portugal é um problema de saúde de grande relevância apresentando umas das maiores taxas de incidência de toda a Europa. Na maioria dos países Ocidentais o cancro gástrico é geralmente diagnosticado em estadios avançados da doença. Contudo, com a generalização da realização de endoscopias digestivas altas, as lesões superficiais neoplásicas gástricas têm vindo a ser mais frequentemente descritas e diagnosticadas. Apesar disso não existem recomendações claras sobre quem deve ser rastreado para a sua presença e apenas recentemente foram publicadas recomendações sobre a avaliação e tratamento destas lesões. Nesta revisão sumariámos a evidência científica atual sobre o diagnóstico, avaliação e o tratamento de lesões superficiais neoplásicas gástricas. Tópicos como rastreio, diagnóstico, avaliação endoscópica, orientação, tratamento, avaliação anatomopatológica e seguimento de doentes com estas lesões serão abordados e áreas de investigação futura serão discutidas. Recomendações baseadas na evidência são feitas quando possível e noutros casos será apresentada a opinião de peritos.
Palavras-Chave: Neoplasias gástricas (MeSH); Mucosectomia; Dissecção submucosa endoscópica; Cancro gástrico inicial; Neoplasia gástrica superficial
Introduction
Gastric adenocarcinoma is still one of the most common and lethal cancers in the world, representing 10% of all deaths from cancer and the fifth leading cancer diagnosis worldwide [1–3]. In Portugal, gastric cancer is the fifth most common and lethal cancer with an incidence of 13 per 100,000 person-years and a mortality of 9 per 100,000 person-years, the highest among European countries [4].
In most Western countries, gastric cancer is generally diagnosed in advanced stages of the disease, in clear contrast to some Eastern countries like Japan. In fact, even though the incidence of early gastric cancer (EGC) diagnosis in Western countries is not really known, a multicenter comparative study found that in North America EGC represented less than 20% of all surgically resected stomachs, in clear contrast to the 50% of surgically resected cancers in Japanese centers [5]. There are several reasons for this geographic variability in the diagnosis of gastric superficial neoplastic lesions, such as the existence of screening programs, training, and more frequent use of dye-based and virtual chromoendoscopy and magnification.
These aspects are very relevant with regard to the treatment of these lesions. Endoscopic resection is considered the standard therapy for gastric superficial neoplastic lesions in Eastern countries like in Japan or Korea, presenting similar efficacy and survival with a potentially better safety profile compared to surgery [6, 7], in contrast to Western countries where surgery is chosen even for initial stages of the disease [8].
Nevertheless, since upper gastrointestinal endoscopy is a widely used procedure, the diagnosis of early lesions appears to be increasing also in Western countries. Moreover, some groups including our own showed that with proper training and skills endoscopic resection techniques could be a first-line treatment also in Western countries [9, 10]. Accordingly, recent European guidelines consider endoscopic resection as a first-line treatment for gastric superficial lesions [11].
In this nonsystematic review, we summarize the current evidence regarding diagnosis and management of gastric neoplastic lesions, establishing evidence-based recommendations.
Diagnosis and Evaluation
When and Who to Screen?
In theory, gastric cancer screening may increase thediagnosis of early lesions and reduce mortality. However, it remains unclear when to perform the screening and who should be screened. Even though several factors and conditions may predispose to gastric cancer, such as prior gastric surgery, family history of gastric cancer, autoimmune gastritis, atrophic/metaplastic gastritis, hereditary and genetic conditions, clear screening indications are not established for many of these situations. In this paper, we will review screening in the general population and in specific groups of patients (Table 1).
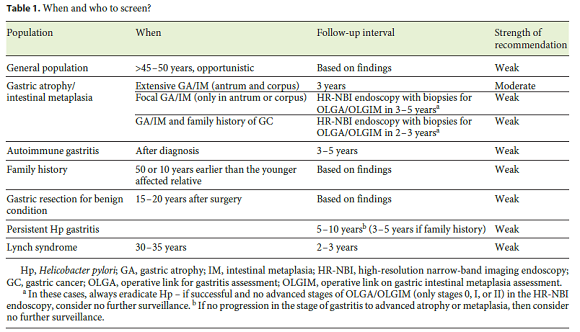
General Population
Screening in the general population can only be recommended in countries with a high gastric cancer incidence such as Eastern and some Latin American countries. In Japan and in Korea, screening is recommended at the age of 40, with endoscopy (or barium X-ray study) being repeated every 2 years [12, 13]. This strategy, particularly with endoscopy instead of X-ray, proved to be cost-effective in these populations [12]. On the other hand, in countries with a low gastric cancer incidence such as the USA, screening is not routinely recommended even in patients considered to be at risk, and the decision to perform endoscopy is then decided on an individual basis [14, 15]. In countries like Portugal (moderate to high incidence), mass screening is difficult to defend. Nevertheless, it is our opinion that a case-finding strategy by proposing a single upper endoscopy after the age of 45–50 years to patients that come back for consultation due to other complaints may be worthwhile not only to increase the detection of early cancers but also to identify people with premalignant conditions (gastric atrophy/metaplasia) that would benefit from further endoscopic surveillance. An idea would be to perform a gastroscopy in patients undergoing screening colonoscopy for colorectal cancer, a strategy that proved to be cost-effective in comparison to commonly performed screening strategies, even in low-risk and high-cost endoscopy countries like the USA [16].
Atrophic/Metaplastic Gastritis
It is clearly established that gastric atrophy and intestinal metaplasia are premalignant lesions (precancerous conditions), with rates of progression to cancer of 0–1.8% and 0–10% per year, respectively [17, 18]. Recent guidelines recommend that patients with extensive atrophy and/or intestinal metaplasia in the antrum and corpus should receive follow-up endoscopy every 3 years [19, 20]. However, no recommendation was made regarding patients that only present atrophy/metaplasia in the antrum, despite the fact that in our own series 25% of gastric lesions appeared in patients that only presented changes in the antrum [10]. Indeed, some advanced stages of atrophy and metaplasia (operative link for gastritis assessment [OLGA]or operative link for intestinal metaplasia [OLGIM]stage III) may only present extensive metaplasia in the antrum and angulus without significant changes in the body [21–23]. Furthermore, recent studies with narrow-band imaging (NBI) suggest that random biopsies may underestimate the degree of atrophy/metaplasia since these changes alternate with normal mucosa even in advanced stages of gastritis [24]. For this reason, patients presenting atrophy/metaplasia solely in the antrum or corpus (random biopsies) could benefit from a highquality endoscopy with NBI every 3–5 years. This endoscopy would allow to endoscopically stage atrophy and intestinal metaplasia, eventually repeating targeted gastric biopsies according to the Sydney-Houston system for calculating OLGA/OLGIM [21]. If there is no progression in the stage of gastritis to advanced atrophy or metaplasia, then no further surveillance could be considered. However, at this moment, this recommendation lacks evidence.
Autoimmune Gastritis
This disorder is also called autoimmune metaplastic atrophic gastritis given the high risk of developing these changes in the gastric body. Even though this condition is associated with an increased gastric cancer risk (2- to 6-fold), no established surveillance recommendation is advocated [25, 26]. Probably, endoscopic surveillance every 3–5 years would be of benefit in these patients. Moreover, this strategy has the advantage of also detecting gastric neuroendocrine tumors of which these patients are at risk [25, 26].
Family History
Family history is clearly recognized as a risk factor for developing gastric cancer [27]. Indeed, guidelines suggest that family history of gastric cancer should be taken into account in the follow-up of patients with precancerous conditions [19]. However, no clear recommendations were made concerning specific patients with a family history of gastric cancer. Moreover, there is some evidence to suggest that gastric premalignant lesions may progress more rapidly in patients with a family history [28]. Taking these factors into account, we believe that patients with advanced stages of atrophy and metaplasia and a family history of gastric cancer in a first-degree relative may benefit from a tighter follow-up strategy, probably with endoscopy every other year. Moreover, a screening endoscopy at the age of 50, or 10 years before the diagnosis of gastric cancer in the family, could be offered to these patients. The cost-utility of these strategies should be further investigated by proper studies.
Partial Gastrectomy
There are insufficient data to recommend endoscopic surveillance in patients with a history of partial gastrectomy because of a benign condition (e.g., peptic ulcer). However, it appears that gastric surgery, by favoring a chronic alkaline reflux into the gastric stump, may facilitate progression of gastritis, atrophy, and metaplasia [29]. Indeed, some studies have shown that these patients are at increased risk of gastric cancer [29, 30]. However, the risk appears to be augmented only 15–30 years after surgery [29, 30]. For this reason, a surveillance endoscopy 15–20 years after surgery with biopsy samples should be considered. If normal histology was found, probably no further surveillance is recommended. If extensive atrophy/ metaplasia is identified, endoscopic surveillance every 2–3 years may be of benefit to these patients.
Persistent Helicobacter pylori Gastritis
With the increasing rates of Helicobacter pylori (Hp) antibiotic resistance, it is predictable that the prevalence of persistent Hp gastritis due to treatment failures will increase. Many patients ask what to do and when to repeat endoscopy after failure to eradicate Hp. The truth is that Hp is the main risk factor for gastric cancer and prospective studies showed that infected patients, even without preneoplastic lesions, may still progress to cancer. Wong et al. [31]showed that the risk of cancer in patients with persistent Hp chronic gastritis without atrophy or intestinal metaplasia is 1% after 7 years (∼ 0.2% patient/year). For these reasons, it is our opinion that patients in whom Hp could not be eradicated should be offered an endoscopy after 5–10 years in order to check the progression of gastritis. If a family history is present, endoscopy should probably be anticipated to 3–5 years. If no progression of gastritis to atrophy and/or metaplasia is observed, then there may be no need to continue endoscopic surveillance. This strategy, however, may lack costeffectiveness and should be investigated further with proper studies before general recommendations can be made.
Hereditary Syndromes
Hereditary syndromes such as familial adenomatous polyposis and lynch syndrome also increase the risk of gastric cancer, and endoscopic surveillance should be considered according to the respective guidelines [32, 33].
Endoscopic Evaluation in Patients with Premalignant Conditions/Lesions
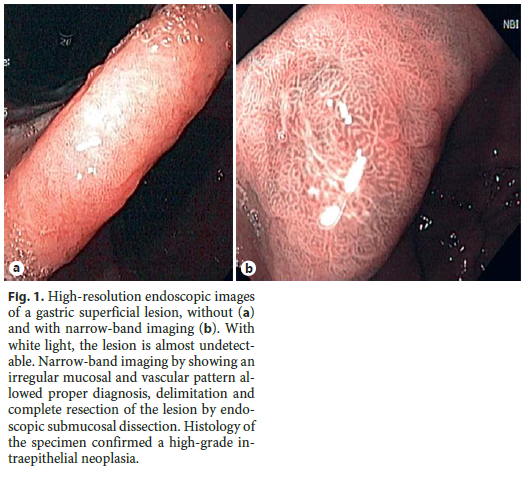
After the histologic diagnosis of a premalignant lesion or EGC (low- or high-grade dysplasia or intramucosal adenocarcinoma), a high-quality endoscopy (with chromoendoscopy – virtual or dye-based) should be offered to the patient, ideally performed by an endoscopist with expertise in the area [11]. It is not uncommon for a gastric superficial neoplasia to be described as an erythema, scar, erosion/ulcer, or a papule. This happens because early neoplastic lesions are rarely detected and thus, most endoscopists are not used to describe these lesions. Moreover, conventional white light endoscopy cannot accurately differentiate these lesions from other benign nonneoplastic lesions [19]. High-resolution NBI (HR-NBI) may help in the diagnosis of these lesions (Fig. 1) [34]. Indeed, our group has shown that a simple NBI classification can be applied with high reproducibility and that an irregular pattern with NBI is highly accurate for the diagnosis of early gastric neoplasia [35]. Chromoendoscopy with indigo carmine or methylene blue with or without magnification may also help in the diagnosis of these lesions; however, this technique is cumbersome and is only available in some centers [36–38]. For these reasons, current guidelines recommend that if a gastric superficial neoplasia is suspected, an experienced endoscopist should perform an upper endoscopy with a scope with HR-NBI (or another digital chromoendoscopy method) [11]. Moreover, the principle of endoscopic treatment is to remove lesions with no or very low risk of lymph node metastasis (LNM), and so virtual chromoendoscopy should be used not only to identify the lesion, but also to predict submucosal invasion and then to correctly delineate it and establish its size. The Paris classification should be used to describe the lesion, since this characteristic along with size and location may also help to predict feasibility and curability of endoscopic resection [39, 40].
Histopathologic Evaluation
Since endoscopic evaluation and patterns are not 100% accurate in the diagnosis of gastric superficial neoplastic lesions, a histologic diagnosis should always be obtained. However, only 1 or 2 fragments of the most suspicious areas, ideally identified and targeted with NBI, should be obtained in order not to induce fibrosis, which may eventually compromise a future resection. The World Health Organization (WHO) classification should be used and the diagnosis of dysplasia/neoplasia should be confirmed by 2 expert gastrointestinal pathologists [19]. However, it should be noticed that if a lesion is identifiable with endoscopy and histology confirms the diagnosis of neoplasia, the lesion should be resected, independently of the grade of dysplasia [19]. So, even a lesion with low-grade dysplasia should be resected, since after complete resection of the lesion there will be a histologic upgrade in almost 1/3 of the lesions [10].
Other Procedures
Currently, there is no other recommended procedure for the evaluation of gastric superficial neoplastic lesions. Some groups routinely use endoscopic ultrasound (EUS). However, a study comparing EUS to expert endoscopic evaluation in order to determine feasibility of endoscopic resection showed that endoscopy alone was better than EUS, since this procedure would upstage many lesions that could be treated by endoscopic resection [41]. Furthermore, there is no evidence that abdominal computed tomography (CT) changes the management of these patients. Indeed, sometimes abdominal CT and/or EUS may create doubts in the treatment by showing perigastric lymph nodes. The clinician might hesitate to use endoscopic treatment even with an endoscopically resectable lesion and most of the times these lymph nodes are not metastatic lymph nodes. For these reasons, guidelines recommend that an expert endoscopic evaluation should be enough for establishing feasibility of endoscopic resection, and that EUS or CT should be avoided [11]. In the worst case scenario, endoscopic resection will provide the best diagnosis and staging, and further staging/treatment can be done if necessary.
Treatment
Endoscopic Mucosal Resection
Endoscopic mucosal resection (EMR) was the first endoscopic treatment that was approved as an alternative to surgery for the treatment of EGC. Two different techniques have been described for the resection of flat/depressed gastric lesions:cap-assisted EMR (EMRc) and rubber band-assisted EMR (EMRb) [42]. In EMRb or ligation- assisted EMR, a standard variceal band ligation device is positioned over the target lesion, generally without prior submucosal injection, and then suction is applied to the lesion. After suctioning the lesion inside the ligation cap, the band is deployed to capture the lesion. The banding device is then removed and a standard electrocautery snare is used to resect the lesion below (preferentially) or above the band. Even though this technique is also described for gastric lesions, its applicability is mainly for esophageal lesions with most experts preferring EMRc for resection of gastric lesions [42]. The technique of EMRc was first described by Inoue et al. [43]. In this technique, a plastic cap is attached to the tip of the endoscope with a snare being prelooped inside the inner aspect of the distal part of the cap. After submucosal injection, the lesion is then suctioned into the cap and the looped snare is used to cut the lesion.
In an early series, EMR was able to cure cancer more than 85% of the times [44, 45]. In selected cases, longterm follow-up of this technique showed 99% diseasespecific survival both at 5 and 10 years [46]. However, the problem with EMR is that the amount of tissue that can be suctioned into the cap is generally low, with most studies showing that lesions bigger than 10–15 mm generally cannot be removed en bloc with negative margins. For this reason, this technique is associated with high rates of local recurrence (almost 30% in some studies) that must be treated either by further endoscopic treatment or surgery [44–48].
Endoscopic Submucosal Dissection
Endoscopic submucosal dissection (ESD) was developed with the main purpose of en bloc removal of larger lesions. It is considered a complex endoscopic technique with a long learning curve and for these reasons only few endoscopists are able to perform this technique, especially in Western countries where gastric cancer incidence is lower and where gastroenterologists are less trained to detect early lesions [42]. Even though the exact technique may vary between experts, some general steps apply to every procedure. After marking the margins of the lesion with an electrocautery device, the technique is initiated with submucosal injection of the lesion. After that, a needle knife is used to make 3–4 small mucosal incisions in order to obtain access to the submucosal layer. Then, the same or a different kind of knife is used to dissect the circumference of the lesion outside the coagulation marks. Thereafter, complete dissection of the lesion is performed with further submucosal injection as needed.
This technique proved to be a great strategy for complete removal with negative margins of gastric lesions, even bigger than 2 cm. Several studies showed that from an oncologic point of view ESD should probably be considered the endoscopic therapy of choice by allowing more than 90% en bloc R0 resections [49, 50].
Surgery
There is no doubt that surgery is the most definitive treatment of EGC with almost every lesion being cured by gastrectomy [51]. The great advantage of surgery is that it allows removal not only of the lesion but also of the regional lymph nodes. However, this may not be necessary in more than 70–80% of the patients, since the risk of LNM may be extremely low in selected EGC [6, 39]. Even though gastrectomy is not considered a high-risk surgery (mortality rates described in the literature of ∼ 1%), it is associated with complications and most of the times can have a negative impact on patients quality of life [51]. Moreover, a proximal lesion surgery may imply a total gastrectomy that is a more aggressive surgery than a distal gastrectomy [52]. Laparoscopic gastrectomy may be a choice for some EGC, eventually with a better safety profile when compared to traditional gastrectomy [53, 54].
Comparison of Treatments
It appears that EMR, ESD and surgery are all associated with high curative rates and long-term cancer-specific survival for the treatment of EGC. The question is, which one should be preferred?
MR versus ESD
Several retrospective Eastern studies, whose data were grouped in 2 meta-analyses, compared EMR with ESD for the treatment of EGC [49, 50]. ESD showed higher en bloc resection rates (92 vs. 52%;OR 9.69;95% CI 7.74–12.13), higher histologic complete resection rates (82 vs. 42%;OR 5.66;95% CI 2.92–10.96), and lower local recurrence (1 vs. 6%;OR 0.10;95% CI 0.06–0.18). These benefits were maintained even for smaller lesions (less than 10 mm). These better outcomes were nevertheless associated with higher procedure times (medium time more than 59.4 min;95% CI 16.8–102) and higher perforation risk (4 vs. 1%;OR 4.67;95% CI 2.77–7.87), although no differences in clinically significant bleeding rates were seen (9% in both groups). The authors concluded that ESD is better than EMR for the treatment of gastric superficial neoplastic lesions, although with a slightly higher risk of perforation. In our Western series, the results were comparable to most Eastern series:higher piecemeal resection (39 vs. 6%, p < 0.001), lower R0 resection (54 vs. 91%, p < 0.001) and consequently higher recurrence rates (15 vs. 3%, p = 0.01) were seen in the EMR group, suggesting that ESD is better than EMR, even though long-term survival rates were not different between the groups. Nevertheless, in our study the safety profile was not different, with 8% of bleeding and 1% perforations in both groups [10].
Endoscopic Treatment versus Surgery
Only few and retrospective studies compare endoscopic treatments with surgery. Two initial EMR studies with a small number of patients and highly selected endoscopic cases did not find any differences in survival [55, 56]. However, even for small lesions, the rates of incomplete resection and recurrence were higher in the EMR group. As predictable, surgery was associated with higher postprocedure morbidity, particularly in elderly patients [55]. In a more recent and larger study, EMR was comparable to surgery not only in survival but also in recurrence [57]. Even though the EMR group had a higher risk of metachronous lesions, all patients were successfully retreated without affecting survival. The complication rate was similar between the groups (∼ 7%), although there was no procedure-related death in the EMR group compared to 2 deaths in the surgery group, and the EMR complications (bleeding) were mostly easily controlled by endoscopy in contrast with some serious complications (wound dehiscence, cholecystitis, and urethral injury) in the surgery group. Moreover, the EMR group had a significantly shorter hospital stay (8 vs. 15 days) and lower cost of care, leading the authors to conclude that EMR has advantages over surgery for the treatment of EGC. In spite of these advantages, almost 30% of the patients in the EMR group were not included in the analysis because they did not have the criteria for complete resection, suggesting that EMR may not be feasible for the treatment of some EGC. Again, this aspect may favor ESD. Indeed, in a cohort comparing ESD to surgery, gastrectomy patients had longer operation times (265 vs. 90 min), a longer hospital stay (10 vs. 3 days) and higher complication rates (33 vs. 5%), with similar oncologic outcomes and survival, leading the authors to conclude that ESD should be the first-line treatment for EGC [58]. Two recent studies also concluded that ESD has many short- and long-term advantages compared to surgery [59, 60]. However, it should be emphasized that in these series only few patients were submitted to laparoscopic gastrectomy, a procedure that appears to have a better safety profile than open gastrectomy [53, 54]. Moreover, even in selected cases with complete resection by ESD, some patients will still need surgery with lymphadenectomy because of noncurative resection (see below). Indeed, in our series, 11% of the patients had an indication for surgery after successful endoscopic resection, even though only 6% of the patients decided to have surgery [10]. Nevertheless, even in these cases, surgery is still an option, with previous ESD not compromising surgery results [61, 62]. In conclusion, even though some patients will still need surgery after ESD, it appears that in selected cases ESD leads to similar oncologic outcomes with a better safety profile compared to surgery.
The Best Choice
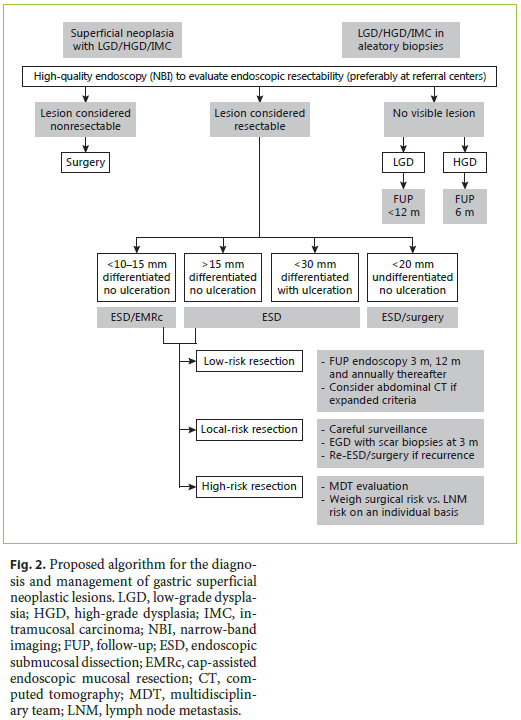
Taking altogether, it is our opinion that when sufficient expertise exists and if the lesion is considered to be endoscopically resectable, then ESD is probably the best choice. Compared to EMR, it allows a more complete oncologic removal of the lesion with negative margins, which might be the difference between curative and noncurative resection (and eventually surgery). Moreover, the safety profile is similar and even though meta-analysis suggests a higher risk of perforation (not in our series), the truth is that most of the gastric ESD perforations are managed conservatively without surgery. Compared to surgery, it theoretically allows the preservation of the stomach, with fewer complications and preserving patients quality of life. However, the patients should always be informed that surgery is the most definitive treatment, that after ESD 10–20% of the patients will still need surgery and that by preserving most of the stomach the risk of new lesions appears to be higher with ESD, approaching 1–2% per patient-years in most series [10]. Exceptions could be patient preference for a more definitive treatment and big (more than 2 cm), depressed lesions in the proximal stomach that are difficult to remove by endoscopy and have a higher risk of LNM. These scenarios, particularly in a younger and fit patient, can make surgery the most compelling treatment. On the other hand, given the very low risk of advanced histology in small nondepressed lesions (Paris IIa, <10–15 mm), particularly if they are located in a difficult position for ESD (e.g., proximal stomach), this could be a good indication for EMR (Fig. 2) [11].
Specimen Histopathologic Evaluation
Handling the Specimen
Resected specimens should be pinned on cork or thick paper immediately after the procedure in order to avoid shrinkage artifacts. Needles should not stretch the specimen too much nor damage the lesion; instead, they should try to maintain its normal size and shape. Completeness of the resection should be confirmed by the identification of the coagulation marks. Needle placement too close to the edges of the specimen or too close to the lesion should be avoided as this may compromise proper histologic evaluation. In cases of piecemeal resection, the endoscopist should try to reconstruct the lesion whenever possible respecting its topography with appropriate fixation onto cork. Diameters of the lesion should be recorded. Colored needles or latex colors may be used for orientation of the specimen, but most of the times this is not necessary since it will not change the management of these patients. After that, the specimen should be placed in 4% buffered formalin overnight remembering that the specimen should be completely covered. Specimens should be sectioned with at least 2-mm intervals to assess tumor involvement in lateral and vertical margins. Two expert gastrointestinal pathologists should make the final histologic diagnosis according to the WHO classification [11, 63–65].
Features to Describe
Histopathologic evaluation is the keystone for deciding if the resection was curative or not and consequently for establishing future management of the patient (Fig. 2). The following characteristics should always be described [11].
Type of Lesion
The lesion should be considered as intraepithelial neoplasia (synonym of dysplasia) of low or high grade, intramucosal invasive carcinoma or submucosal invasive carcinoma. It should be noticed that, contrarily to the colon, intramucosal carcinoma is invasive and malignant and has the potential of LNM. Differentiation The grade of differentiation of carcinomas should be divided into grade 1 (well differentiated), 2 (moderately differentiated), and 3 (poorly differentiated or diffuse type). From a clinical point of view, the important division is between grade 1/2 and grade 3, since management will be different.
Size of Lesion
The macroscopic size of the lesion should be measured in centimeters, since this may influence the management of the patient.
Ulcerative Findings
It should be recorded if there are ulcerative findings in the histopathologic evaluation, since its presence can influence the resection classification.
Lymphovascular Invasion
This should always be recorded as positive (LV1) or negative (LV0) in lesions with carcinoma (not necessary in dysplasia), since this is the most important factor for predicting LNM.
Perineural Invasion
Even though the prognostic role of perineural invasion is controversial, particularly in EGC, this should be recorded.
Depth of Submucosal Invasion
This should be recorded in micrometers. The important factor is to establish if the depth of submucosal invasion is more or less than 500 μm.
Resection Margins
Resection margins should be divided into negative (0) and positive (1) and into horizontal (Hm) and vertical (Vm) margins, since the clinical meaning of Hm1 and Vm1 is different. There are no studies showing which distance should be considered a safe margin. Generally, if tumor (dysplasia or carcinoma) is not present in the margins, independently of the distance, it should be considered a negative margin (Hm0, Vm0). If tumor is present (or indeterminate), it should be considered a positive margin and the resection should be considered at least an Rx resection (Rx if Hm1, Vm0 and R1 if Vm1).
Management after Specimen Histopathology Result
Type of Resection
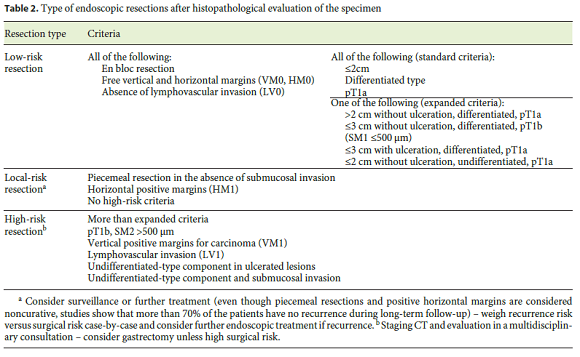
Traditionally, the terms curative and noncurative were used to refer to the type of resection. However, definitions vary between studies, and even in the worst scenario of a complete noncurative resection, more than 70% of the patients are in fact cured. For these reasons, current European recommendations homogenized definitions and considered 3 types of resection (Table 2) [11].
Low-Risk Resection
In this case, the risk of distant or local recurrence is extremely low (<1%) and no further treatment is recommended (given that recurrence risk is lower that surgical mortality risk).
Local-Risk Resection
In this case, the risk of distant disease is extremely low (<1%); however, the risk of local recurrence may be as high as 30%. Generally, it happens after piecemeal resection or positive horizontal margins. In these cases, tight endoscopic surveillance and eventually another endoscopic treatment are recommended.
High-Risk Resection
In this case, there is a small but real risk for distant disease (>2%), theoretically higher than the risk of surgery. Staging procedures (e.g., CT), multidisciplinary evaluation, and consideration of adjuvant treatment (surgery) are recommended.
Low-Risk Resection (Curative Criteria)
Two different curative criteria are described in the literature, even though the risk of LNM appears almost zero in either group. Initially, only the standard/traditional criteria were considered curative because there were only few studies evaluating predictive factors of LNM in EGC and EMR only allowed en bloc removal of small (less than 2 cm) lesions. With the development and dissemination of ESD that allowed en bloc removal of bigger lesions, several studies showed that other additional factors could be considered as curative (expanded indications) [6, 66, 67].
Standard Criteria
Standard criteria include an en bloc R0 resection (Hm0, Vm0) of a dysplastic lesion or intramucosal carcinoma, with less than 20 mm, no ulceration and no lymphovascular involvement. The risk of LNM is 0% (95% CI 0–0.3).
Expanded Criteria
Expanded criteria include an en bloc R0 resection (Hm0, Vm0) of any size of an intramucosal well-differentiated carcinoma (95% CI 0–0.4), less than 30 mm of an ulcerated intramucosal carcinoma (95% CI 0–0.3), submucosal infiltration less than 500 μm (Sm1) in a tumor less than 30 mm (95% CI 0–2.5), or undifferentiated/diffuse intramucosal carcinoma with less than 20 mm (95% CI 0–1). The risk of LNM with these criteria is also 0%.
Current guidelines recognize that these criteria are in fact criteria of low-risk resection;however, given the low number of cases described in the literature, the upper limit of the 95% CI can be as high as 2.5% for the expanded criteria and thus abdominal CT is generally recommended, even though there is no evidence to support this strategy [11].
High-Risk Resection (Noncurative Criteria)
If any of the following features is observed, the patient should be proposed for surgery:intramucosal cancer bigger than 3 cm but with ulcerative findings (LNM risk of 2% if well differentiated and 7% if poorly differentiated); poorly differentiated intramucosal carcinoma bigger than 2 cm (LNM risk of 2% if 2–3 cm and 7% if >3 cm); poorly differentiated carcinoma with submucosal invasion (>7% depending on depth of invasion and size);Sm1 well-differentiated tumors bigger than 3 cm (LNM risk of 3%);submucosal infiltration deeper than 500 μm (at least Sm2;LNM risk >10%, depending on size of the lesion) and lymphovascular invasion (LNM risk >21%) [6, 66, 67]. Positive vertical margins (Vm1) for carcinoma are also a criterion for high-risk resection (see below). However, it should be noticed that many of these patients are old and have significant comorbidities;thus, the risk of gastrectomy should always be balanced against the LNM risk.
Local-Risk Resection (Positive Margins)
There is no consensus regarding the management when a lesion with otherwise low-risk criteria is not resected en bloc or presents with positive margins. Several studies show that when apparently there was no lesion in the ulcer at the end of the procedure, even with piecemeal resection, and/or there were clearly positive histologic margins, the risk of recurrence is still only about 10–30%, meaning that even in these cases, about 70–90% of the patients will be cured [47, 68]. Moreover, it appears that most of these incomplete resections are amenable to further endoscopic treatment, without the need of surgery [47, 69–71]. However, evidence shows that Hm1 is clearly distinct from Vm1, since a positive Vm is associated with a higher recurrence rate (>40%, most of the times not amenable to further endoscopic treatment) as well as with some risk of LNM (>5%) [48, 72]. These findings were confirmed in other series;thus, it is our opinion that, and in agreement with Japanese and European guidelines, patients with positive lateral margins in the absence of positive vertical margins, undifferentiated tumor, and submucosal or lymphovascular invasion can be managed with further endoscopic surveillance or therapy, without the need of surgery [6, 11, 73]. If the vertical margins are positive, with the exception of lesions only with dysplasia, this should be a criterion for high-risk resection, and surgery is recommended depending on the clinical condition of the patient.
Uncertain Areas
Another controversial issue is when the tumor presents a mixed pattern (well differentiated with some areas of isolated cells/diffuse pattern). Given the lack of evidence regarding these tumors, guidelines do not consider these situations. Even though some authors suggest that the percentage of the type of tumor should determine the final diagnosis (e.g., if more than 50% are diffuse cells, it is considered as an undifferentiated tumor and if less than 50%, it is considered as a differentiated tumor), there is no evidence to support further management. For this reason, we suggest that even small areas of isolated cells should categorize these tumors as undifferentiated and they should be treated accordingly, at least until further evidence is provided. Regarding perineural invasion in the absence of high-risk criteria, a feature that rarely presents in isolation and is rarely studied, there is no evidence to guide treatment. A recent study suggests that in the absence of lymphovascular invasion, perineural invasion does not increase the risk of LNM [74]. So, it is our opinion that future studies should further investigate this aspect and that this feature, when present in isolation, should not interfere with the decision about the necessary treatment.
Follow-Up
If the patient is not considered to have an indication for gastrectomy then endoscopic surveillance should be initiated. Eradication of Hp should be performed since it has been shown that it might reduce the risk of metachronous lesions [19]. Even though no study compared different strategies of follow-up, one prospective study showed that endoscopic follow-up is cost-effective, since it allows the detection and management of new lesions without the need for surgery [75]. This happens because after endoscopic resection the rate of new lesions (synchronous, recurrent, or metachronous) can be as high as 1–3% per year in the first 5 years [10, 75]. Moreover, it appears that the first 2 endoscopies are the ones that detect more lesions, particularly synchronous lesions not previously detected, and recurrences [10, 75]. For these reasons, we suggest 2 endoscopies in the first year (at 3–6 months and then at 9–12 months) and then annually. It should be noticedthat in the first 1–3 months, sometimes the scar appears elevated with granulation tissue and so it might be difficult to distinguish between recurrence and granulation tissue, both endoscopically and histologically [76]. For this reason, we do not recommend to do the first follow-up endoscopy before the 3 months. Concerning biopsies, we do not recommend scar biopsies on a routine basis. Instead, we recommend high-quality endoscopy (HR-NBI) and taking biopsies only if suspicious areas are seen. Again, if the resection was R0 and if we see some polypoid aspect of the scar, particularly when it suggests inflammation/granulation tissue, we avoid biopsies at least in the first endoscopy because the probability of being a recurrence is extremely low and by taking biopsies there is a risk of increasing the size of the hyperplastic polyp [76]. On the other hand, if the resection was Rx (local risk) then taking 1 or 2 fragments from the scar should be considered. Concerning other evaluations, there is no evidence to support any other procedure. After a resection with expanded criteria, an abdominal CT can be considered even though the probability of detecting metastatic disease is extremely low. Moreover, the periodicity of radiologic surveillance is not established. On the other hand, a resection with high-risk criteria should be followed by all the examinations recommended for the staging of gastric cancer, including CT [11].
Conclusions and Areas of Future Research
With the widespread use of endoscopy and the new techniques available to detect these lesions at an early stage (e.g. HR-NBI), EGC is being increasingly recognized in Western countries.
Despite there being some recommendations about follow-up of preneoplastic conditions, evidence is scarce about the utility of screening asymptomatic populations for gastric cancer and for these preneoplastic conditions. In our country, a region with a moderate to high incidence of gastric cancer, we suggest to opportunistically screen patients older than 45–50 years and we make recommendations about screening and follow up patients with known risk factors for gastric cancer [77].
Gastric superficial lesions/EGC can be managed either endoscopically or surgically, with endoscopic resection being considered a first-line therapy. In this article, we review comparative studies of EMR, ESD and gastrectomy in the treatment of EGC, which show similar efficacy between ESD and gastrectomy and a trend for a better safety profile with ESD (compared to gastrectomy). So, in line with current European guidelines, we suggest ESD as the first-line treatment [11].
In order to overcome some lack of evidence in this area, we hope that future studies can clarify the optimal strategy of follow-up for patients with preneoplastic conditions and comparatively evaluate clinical outcomes of gastrectomy and endoscopic resection in the treatment of EGC as well as evaluate which is the more cost-effective therapy. Besides, future identification of clinical, endoscopic, and histologic/molecular characteristics associated with a higher incidence of metachronous lesions or a more aggressive course of the disease may influence treatment, perhaps guiding us to select the patients more suitable for a surgical management ab initio.
In this article, we summarize every step in the management of a patient with EGG. Although some of the recommendations are based on expert opinion, it is our view that these can guide clinicians in this developing field where evidence-based recommendations are difficult to make at this time.
References
1 Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al: Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [ Links ]
2 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D: Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [ Links ]
3 Jemal A, Siegel R, Xu J, Ward E: Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [ Links ]
4 Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al: GLOBOCAN 2012. Cancer incidence and mortality worldwide. http://globocan.iarc.fr 2015. [ Links ]
5 Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF: Is gastric carcinoma different between Japan and the United States? Cancer 2000;89:2237–2246. [ Links ]
6 Japanese Gastric Cancer Association: Japanese gastric cancer treatment guidelines 2010 (ver 3). Gastric Cancer 2011;14:113–123. [ Links ]
7 Jung HY: Endoscopic resection for early gastric cancer: current status in Korea. Dig Endosc 2012;24(suppl 1):159–165. [ Links ]
8 Ajani JA, Bentrem DJ, Besh S, DAmico TA, Das P, Denlinger C, et al: Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531–546. [ Links ]
9 Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L: A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc 2009;69:350–355. [ Links ]
10 Pimentel-Nunes P, Mourao F, Veloso N, Afonso LP, Jacome M, Moreira-Dias L, et al: Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy 2014;46:933–940. [ Links ]
11 Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al: Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829–854. [ Links ]
12 Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC: Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013; 14:2533–2540. [ Links ]
13 Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al: Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008;9:279–287. [ Links ]
14 Leja M, You W, Camargo MC, Saito H: Implementation of gastric cancer screening – the global experience. Best Pract Res Clin Gastroenterol 2014;28:1093–1106. [ Links ]
15 Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M: Gastric cancer:prevention, screening and early diagnosis. World J Gastroenterol 2014;20:13842–13862. [ Links ]
16 Gupta N, Bansal A, Wani SB, Gaddam S, Rastogi A, Sharma P: Endoscopy for upper GI cancer screening in the general population:a cost-utility analysis. Gastrointest Endosc 2011;74:610–624.e2. [ Links ]
17 Lahner E, Bordi C, Cattaruzza MS, Iannoni C, Milione M, Delle Fave G, et al: Long-term follow-up in atrophic body gastritis patients:atrophy and intestinal metaplasia are persistent lesions irrespective of Helicobacter pylori infection. Aliment Pharmacol Ther 2005;22:471–481. [ Links ]
18 de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al: Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945–952. [ Links ]
19 Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, OConnor A, et al: Management of precancerous conditions and lesions in the stomach (MAPS):guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. [ Links ]
20 Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, OConnor A, et al: Management of precancerous conditions and lesions in the stomach (MAPS):guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Virchows Arch 2012;460:19–46. [ Links ]
21 Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ , et al: The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–1158. [ Links ]
22 Isajevs S, Liepniece-Karele I, Janciauskas D, Moisejevs G, Funka K, Kikuste I, et al: The effect of incisura angularis biopsy sampling on the assessment of gastritis stage. Eur J Gastroenterol Hepatol 2014;26:510–513. [ Links ]
23 Varbanova M, Wex T, Jechorek D, Rohl FW, Langner C, Selgrad M, et al: Impact of the angulus biopsy for the detection of gastric preneoplastic conditions and gastric cancer risk assessment. J Clin Pathol 2016;69:19–25. [ Links ]
24 Lage J, Pimentel-Nunes P, Figueiredo PC, Libanio D, Ribeiro I, Jacome M, et al: Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: do we still need biopsies? Scand J Gastroenterol 2016;51:501–506. [ Links ]
25 Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, et al: Cancer risk after pernicious anemia in the US elderly population. Clin Gastroenterol Hepatol 2015;13:2282–2289.e1–e4. [ Links ]
26 Vannella L, Lahner E, Osborn J, Annibale B: Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375–382. [ Links ]
27 Yaghoobi M, Bijarchi R, Narod SA: Family history and the risk of gastric cancer. Br J Cancer 2010;102:237–242. [ Links ]
28 Marcos-Pinto R, Dinis-Ribeiro M, Carneiro F, Wen X, Lopes C, Figueiredo C, et al: First-degree relatives of early-onset gastric cancer patients show a high risk for gastric cancer: phenotype and genotype profile. Virchows Arch 2013;463:391–399. [ Links ]
29 Sinning C, Schaefer N, Standop J, Hirner A, Wolff M: Gastric stump carcinoma – epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol 2007;33:133–139. [ Links ]
30 Lagergren J, Lindam A, Mason RM: Gastric stump cancer after distal gastrectomy for benign gastric ulcer in a population-based study. Int J Cancer 2012;131:E1048–E1052. [ Links ]
31 Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al: Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–194. [ Links ]
32 Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al: Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812–823. [ Links ]
33 Vasen HF, Moslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al: Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–713. [ Links ]
34 Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu S, et al: Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer 2016;19:543–552. [ Links ]
35 Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, et al: A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012;44:236–246. [ Links ]
36 Areia M, Amaro P, Dinis-Ribeiro M, Cipriano MA, Marinho C, Costa-Pereira A, et al: External validation of a classification for methylene blue magnification chromoendoscopy in premalignant gastric lesions. Gastrointest Endosc 2008;67:1011–1018. [ Links ]
37 Dinis-Ribeiro M: Chromoendoscopy for early diagnosis of gastric cancer. Eur J Gastroenterol Hepatol 2006;18:831–838. [ Links ]
38 Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Moreira-Dias L: Feasibility and cost-effectiveness of using magnification chromoendoscopy and pepsinogen serum levels for the follow-up of patients with atrophic chronic gastritis and intestinal metaplasia. J Gastroenterol Hepatol 2007;22:1594–1604. [ Links ]
39 The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–S43. [ Links ]
40 Hirasawa K, Kokawa A, Kou R, Oka H, Maeda S, Tanaka K: Determining early gastric cancer lesions appropriate for endoscopic submucosal dissection trainees: a proposal related to curability. Dig Endosc 2012;24(suppl 1):143–147. [ Links ]
41 Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS: Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42:705–713. [ Links ]
42 Asge Technology C, Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, et al: Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc 2008;68:11–18. [ Links ]
43 Inoue H, Endo M, Takeshita K, Yoshino K, Muraoka Y, Yoneshima H: A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc 1992;6:264–265. [ Links ]
44 Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, et al: Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy 1994;26:352–358. [ Links ]
45 Hiki Y, Shimao H, Mieno H, Sakakibara Y, Kobayashi N, Saigenji K: Modified treatment of early gastric cancer: evaluation of endoscopic treatment of early gastric cancers with respect to treatment indication groups. World J Surg 1995;19:517–522. [ Links ]
46 Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, et al: Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer 2006;9:88–92. [ Links ]
47 Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, et al: Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc 2012;26:72–78. [ Links ]
48 Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, et al: Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer 2005;8:149–154. [ Links ]
49 Lian J, Chen S, Zhang Y, Qiu F: A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763–770. [ Links ]
50 Park YM, Cho E, Kang HY, Kim JM: The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 2011;25:2666–2677. [ Links ]
51 Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M: Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 2008;95:1131–1135. [ Links ]
52 Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, et al: Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg 2014;38:1100–1106. [ Links ]
53 Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L: Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg 2012;256:39–52. [ Links ]
54 Zhang CD, Chen SC, Feng ZF, Zhao ZM, Wang JN, Dai DQ: Laparoscopic versus open gastrectomy for early gastric cancer in Asia: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2013;23:365–377. [ Links ]
55 Etoh T, Katai H, Fukagawa T, Sano T, Oda I, Gotoda T, et al: Treatment of early gastric cancer in the elderly patient: results of EMR and gastrectomy at a national referral center in Japan. Gastrointest Endosc 2005;62:868–871. [ Links ]
56 Kim HS, Lee DK, Baik SK, Kim JM, Kwon SO, Kim DS, et al: Endoscopic mucosal resection with a ligation device for early gastric cancer and precancerous lesions: comparison of its therapeutic efficacy with surgical resection. Yonsei Med J 2000;41:577–583. [ Links ]
57 Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim do H, et al: EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 2011;73:942–948. [ Links ]
58 Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, et al: Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 2012;26:3584–3591. [ Links ]
59 Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, et al: Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2016;30:3762–3773. [ Links ]
60 Song WC, Qiao XL, Gao XZ: A comparison of endoscopic submucosal dissection (ESD) and radical surgery for early gastric cancer: a retrospective study. World J Surg Oncol 2015;13:309. [ Links ]
61 Jung H, Bae JM, Choi MG, Noh JH, Sohn TS, Kim S: Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg 2011;98:73–78. [ Links ]
62 Kwon HY, Hyung WJ, Lee JH, Lee SK, Noh SH: Outcomes of laparoscopic gastrectomy after endoscopic treatment for gastric cancer: a comparison with open gastrectomy. J Gastric Cancer 2013;13:51–57. [ Links ]
63 Dinis-Ribeiro M, Chaves P; Sociedade Portuguesa de Endoscopia Digestiva e Divisao Portuguesa da Academia Internacional de Patologia:Portuguese Society of Digestive Endoscopy:recommendations for endoscopic mucosal resection. Endoscopy 2008;40:622–623. [ Links ]
64 Dixon MF: Gastrointestinal epithelial neoplasia:Vienna revisited. Gut 2002;51:130–131. [ Links ]
65 Dixon MF, Genta RM, Yardley JH, Correa P: Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–1181. [ Links ]
66 Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I: Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010; 97:868–871. [ Links ]
67 Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al: Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219–225. [ Links ]
68 Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, et al: Risk of recurrent gastric cancer after endoscopic resection with a positive lateral margin. Endoscopy 2014;46:273–278. [ Links ]
69 Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, et al: Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy 2012;44:1114–1120. [ Links ]
70 Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, et al: Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg 2008;95:1495–1500. [ Links ]
71 Yokoi C, Gotoda T, Hamanaka H, Oda I: Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 2006;64:212–218. [ Links ]
72 Figueiredo PC, Pimentel-Nunes P, Libanio D, Dinis-Ribeiro M: A systematic review and meta-analysis on outcomes after Rx or R1 endoscopic resection of superficial gastric cancer. Eur J Gastroenterol Hepatol 2015;27:1249–1258. [ Links ]
73 Yoon H, Kim SG, Choi J, Im JP, Kim JS, Kim WH, et al: Risk factors of residual or recurrent tumor in patients with a tumor-positive resection margin after endoscopic resection of early gastric cancer. Surg Endosc 2013;27:1561–1568. [ Links ]
74 Ahmad R, Setia N, Schmidt BH, Hong TS, Wo JY, Kwak EL, et al: Predictors of lymph node metastasis in Western early gastric cancer. J Gastrointest Surg 2016;20:531–538. [ Links ]
75 Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, et al: Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013;62:1425–1432. [ Links ]
76 Mitsuhashi T, Lauwers GY, Ban S, Mino-Kenudson M, Shimizu Y, Ogawa F, et al: Postgastric endoscopic mucosal resection surveillance biopsies: evaluation of mucosal changes and recognition of potential mimics of residual adenocarcinoma. Am J Surg Pathol 2006; 30:650–656. [ Links ]
77 Areia M, Pimentel-Nunes P, Marcos-Pinto R, Dinis-Ribeiro M: Gastric cancer: an opportunity for prevention. Acta Med Port 2013;26:627–629. [ Links ]
* Corresponding author.
Dr. Pedro Pimentel-Nunes
Department of Gastroenterology, Portuguese Oncology Institute
Rua Dr António Bernardino de Almeida
PO–4200-072 Porto (Portugal)
E-Mail pedronunesml@gmail.com
Received:April 8, 2016;Accepted after revision:July 3, 2016














