Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.1 Lisboa fev. 2017
https://doi.org/10.1159/000450898
CLINICAL CASE STUDIES
Superior Mesenteric Artery Syndrome: Weight Loss Can Be a Problem, Weight Gain Can Be a Solution
Síndrome da Artéria Mesentérica Superior: Quando a Perda Ponderal Se Torna um Problema e o Aumento de Peso a Solução!
Ana Alvarengaa, Céu Espinheirab, Paula Guerrab, Maria Garciaa, Mariana Abreub, Miguel Camposa
a Pediatric Surgery Department and b Pediatric Department, Centro Hospitalar São João, Porto, Portugal
* Corresponding author.
ABSTRACT
Introduction: Superior mesenteric artery syndrome (SMAS) is a rare acquired disorder, which in the present case had an acute and unusual way of presentation. Case Report: We present a 17-year-old female with nausea, vomiting, and intense epigastric pain. In the previous 6 months, she had lost 42% of her body weight. The echography showed a distended stomach that reached the pelvis, and the nasogastric tube that was placed drained 2,000 mL. A computed tomography scan confirmed the SMAS diagnosis. She started a hypercaloric fractionated meal diet, prokinetics, and postural measures. After the 1-year follow-up the patient is asymptomatic. Conclusion: This acute presentation is rare but lifethreatening due to the possibility of gastric rupture. Medical management is possible in the majority of cases, and surgery is needed only in the refractory ones.
Keywords: Adolescents; Diet; Superior mesenteric artery syndrome;·Weight gain; Weight loss
RESUMO
Introdução: A síndrome da artéria mesentérica superior (SAMS) é uma patologia rara sendo o caso clínico que publicamos relevante devido à sua apresentação aguda e grave. Caso Clínico: Adolescente de 17 anos, com quadro de náuseas, vómitos e dor epigástrica intensa. História pregressa de perda ponderal correspondente a 42% do peso corporal em 6 meses. Realizou ecografia abdominal que mostrou distensão do estômago até à pelve. Colocada sonda nasogástrica com drenagem de 2,000 mL de conteúdo alimentar. Confirmado diagnóstico de SMAS por TC, iniciou regime hipercalórico fracionado via oral, procinéticos e medidas posturais com resolução do quadro. A doente é seguida em consulta externa há um ano e está assintomática. Conclusão: Esta apresentação aguda é rara e grave devido à possibilidade de rutura gástrica. O tratamento médico é possível na maioria das vezes, estando a cirurgia reservada para os casos refratários.
Palavras-Chave: Adolescente; Dieta; Ganho de peso; Perda de peso;·Síndrome da artéria mesentérica superior
Introduction
Superior mesenteric artery syndrome (SMAS) is an acquired disorder that results from compression of the third portion of the duodenum between the abdominal aorta and the superior mesenteric artery. Retroperitoneal fat pad loss is thought to be the main cause of acute angulation and SMAS symptoms [1]. Its incidence varies from 0.013 to 0.3% [2]. A case of SMAS successfully treated with a conservative approach is presented here.
Clinical Case
A 17-year-old female was transferred to our hospital due to acute gastric distension and intestinal occlusion. A week before the transfer, she had presented to the emergency room with nausea, emesis, and intense epigastric pain. She could not tolerate solids or fluids, and the pain worsened after food ingestion. In the prior 6 months, she had lost 42% of her body weight (86–50 kg) due to exercise and a self-conducted diet. Physical examination was significant for pain in the upper abdominal quadrants with no palpable masses. Laboratory studies (blood count, hormonal studies, ionogram, and renal and hepatic function) and cerebral computed tomography (CT) scan were normal. The symptoms were paroxystic but worsened with time. During the hospital stay (7 days), she lost an additional 5 kg. An abdominal ultrasound was performed and revealed a dilated stomach (from the left hypochondrium to the pelvis), the first and second part of the duodenum suggesting intestinal occlusion. A nasogastric tube was placed and drained more than 2 L of biliary content with symptomatic relief.
When observed at our hospital (hours later), she was not in acute distress, her body mass index was 17.9, and her vital signs were within the normal range. She had epigastric tenderness and hyperactive bowel sounds without palpable masses or organomegalies. The remaining examination was unremarkable. Complete blood count, serum electrolytes, urea, creatinine, and C-reactive protein were normal; aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase were slightly elevated (42 U/L, 62 U/L, and 58 U/L, respectively). An abdominal CT scan revealed an acute angle of 17.1° between the aorta and the superior mesenteric artery (Fig. 1), narrowing the third portion of the duodenum.These findings established the diagnosis of SMAS.
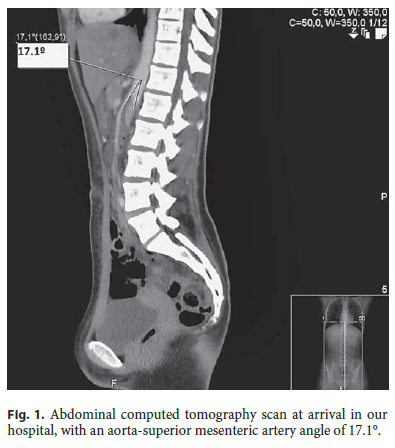
A hypercaloric fractionated meal diet (polymeric liquids), metoclopramide, and postural measures (left decubitus during 30 min after meals) were initiated. The diet was well tolerated and titrated slowly up to soft diet, while being monitored for refeeding syndrome. After 17 days, she gained 6 kg and could already ingest solid food. She was discharged without pain and tolerating the prescribed diet. She stayed as an outpatient in the nutrition and pediatric surgery clinics. At the 1-year follow-up she was doing well with stable weight (58.25 kg, body mass index 21.7) and without symptoms.
Discussion
The SMA leaves the aorta at the level of the first lumbar vertebra, creating an acute angle through which the third portion of the duodenum passes [1]. When the angle measures less than 20°, SMAS symptoms can appear due to duodenum compression and consequent intestinal occlusion [1]. SMAS syndrome is very rare but can have many predisposing factors, such as anatomic variations, postsurgical reasons (external compression, spinal correction surgery, and abdominal surgery), trauma, local pathology (malignancy, aortic aneurysm, chronic inflammation, and adhesions), and loss of mesenteric fat. The predisposing factors are associated with each other and may cause SMAS. The decrease in the fat pad can also occur in patients dependent on tube feeding who have been underfed, after gastric bypass surgery and after malabfat was due to a fasting diet and excessive exercise. SMAS symptoms are often intermittent and experienced in unpredictable ways. These characteristics in combination with the low prevalence of the disease lead to a difficult diagnosis [3]. As in our patient, diagnosis is often delayed because of the lack of specificity of symptoms including postprandial nausea, early satiety, abdominal pain, and emesis immediately after meals (30 min to 1 h), frequently bilious and sometimes with partially digested food [2]. The symptoms may be quite mild, gradually increasing over weeks or suddenly worsening when a critical weight is reached. An acute presentation is more common in cases associated with rapid growth or rapid weight loss, as in our patient. Also, as in our patients first week of hospitalization, symptoms can be severe enough to cause anorexia and food aversion, worsening weight loss and exacerbating the duodenal compressionand symptoms.
The diagnosis is made by radiologic, angiographic, ultrasonic, and endoscopic studies. Abdominal X-ray can only show gastric distension at best. The upper gastrointestinal series contrast study has been the standard diagnosis procedure for decades (showing dilated first and second portions of the duodenum with abrupt vertical obstruction of the third portion); it consists of antiperistaltic waves of contrast moving away from the obstruction, a 4- to 6-h delay in transit of contrast to the jejunum, with relief of the obstruction through postural changes. Measuring the aortomesenteric angle in the abdominal angio-CT of a patient with symptoms can be diagnostic. In our patient, the diagnosis was made by an angio-CT scan after a SMAS suspicion due to clinical signs and acute gastric distention [4] discovered in the abdominal ultrasonography. In fact, CT is the exam that diagnoses this condition in about 94% of cases nowadays [2].
Patients can be treated medically or surgically. We advocate initial medical treatment, since it is least invasive for the patient and is connected with less morbidity. Treatment of SMAS is usually conservative [5, 6]. Acute management is focused on bowel decompression and then maintenance of the fluid and electrolyte balance and nutritional support or rehabilitation. Weight gain is generally encouraged to increase the mesenteric fat pad and thereafter the aortomesenteric angle, thereby preventing duodenal compression.
The fractionated hypercaloric meal diet with prokinetic drugs and postural measures (lying prone in knee-chest position or left-sided lying after meals) [1] can lead to weight gain and resolve the symptoms, as happened in our patient. A proton pump inhibitor can protect the gastric mucosa in this stressing situation, with slow gastric emptying times, and contribute to decreasing the patients dyspepsia [5]. In some cases, oral feeding is not tolerated, and a jejunal tube with enteral nutrition is needed. Parenteral feeding, combined with enteral feeding or alone, is needed in patients with an unsuccessful jejunal feeding plan.
In a study from 2000 to 2009 in 7 institutions, the medical management success rate was 71.3%, and the recurrence rate 15.8% [2]. Advances in both enteral and parenteral nutrition have dramatically impacted the medical management of SMAS.
Patients with SMAS after drastic weight loss due to inadequate intake are at risk of refeeding syndrome during medical treatment. Due to starvation, the body is in a catabolic state causing electrolyte depletion. When they start to eat gradually increased amounts of food, the body switches into an anabolic state, and cells start to take up potassium, phosphorous, and magnesium with huge fluid shifts. The patient can experience symptomatic hypophosphatemia and even rhabdomyolysis, congestive heart failure, and death. These risks make medical hospital treatment necessary, as well as close electrolyte and cardiac monitoring with electrolyte repletion as needed.
After 4–6 weeks, if the medical approach does not lead to weight gain, surgical treatment should be considered. Surgical management has a high success rate (92.9%, with laparoscopic duodenojejunostomy being the most common procedure) [2]. Surgical treatment of SMAS is chosen in chronic cases, patients with peptic ulcer (due to a greater perforation risk), or patients that do not resolve with conservative measures. The duodenojejunostomy is a relatively simple procedure, and the success rate for duodenojejunostomy has been reported to be between 80 and 90%. The gastrojejunostomy has been abandoned due to the risks of bile reflux gastritis; it is generally employed only when the duodenum has significant ulceration, making it unsafe to perform a duodenojejunostomy. In the duodenal derotation, the ligament of Treitz is cut, allowing the third portion of the duodenum to be moved inferiorly and laterally away from the aortomesenteric angle, removing the risk of duodenal compression [7]. This is the recommended procedure in some institutions, since it has a 75% success rate and does not require an anastomosis. Nowadays, this procedure can be done by laparoscopy with good results, immediate resolution of bowel occlusion, minimum pain, and minimum scar [8–9].
Conclusion
SMAS is a rare condition and usually appears in association with chronic and consumptive diseases but should be considered in the differential diagnosis of abdominal symptoms in a teenager who has lost significant weight. SMAS can be successfully managed medically, focusing on gastric decompression, adequate nutrition, and proper positioning after meals, thus avoiding surgery which is reserved for refractory cases.
References
1 Gebhart T: Superior mesenteric artery syndrome. Gastroenterol Nurs 2015;38:189–193. [ Links ]
2 Lee TH, Lee JS, Jo Y, Park KS, Cheon JH, Kim YS, et al: Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg 2012;16:2203–2211. [ Links ]
3 Gould R, Sandstrom CK, Strote J: Identification of superior mesenteric artery syndrome from vascular angle measurement. J Emerg Med 2015;49:35–36. [ Links ]
4 Sato H, Tanaka T: Acute gastric dilatation due to a superior mesenteric artery syndrome: an autopsy case. BMC Gastroenterol 2014;14:37. [ Links ]
5 Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB: Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B 2014;23:312–318. [ Links ]
6 Naseem Z, Premaratne G, Hendahewa R: Less is more: non operative management of short term superior mesenteric artery syndrome. Ann Med Surg (Lond) 2015;4:428–430. [ Links ]
7 Alsulaimy M, Tashiro J, Perez E, Sola J: Laparoscopic Ladds procedure for superior mesenteric artery syndrome. J Pediatr Surg 2014;49:1533–1535. [ Links ]
8 Pottorf BJ, Husain FA, Hollis HW, Lin E: Laparoscopic management of duodenal obstruction resulting from superior mesenteric artery syndrome. JAMA Surg 2014;149319–1322. [ Links ]
9 Massoud WZ: Laparoscopic management of superior mesenteric artery syndrome. Int Surg 1995;80:322–327. [ Links ]
Statement of Ethics
The information in this paper is presented after the informed consent of the patient and parents was obtained. It respects confidentiality and anonymity of the patient and reports true facts with quality and integrity.
Disclosure Statement
The authors declare that they have no conflicts of interest.
* Corresponding author.
Dr. Ana Alvarenga
Pediatric Surgery Department
Centro Hospitalar São João
PT–4200-319 Porto (Portugal)
E-Mail anasofialvarenga@gmail.com
Received: June 27, 2016; Accepted after revision: August 30, 2016














