Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.4 Lisboa ago. 2017
https://doi.org/10.1159/000453302
CLINICAL CASE STUDY
Gastric Adenomyoma: The Unexpected Mimicker
Adenomioma Gástrico: O Inesperado Simulador
Marcela Adriana Duran Álvareza, Juan Ramón Gómez Lópezb, Teresa Guerra Garijoc
Departments of aPathology, bSurgery, and cRadiology, Medina del Campo Hospital, Valladolid, Spain
* Corresponding author.
ABSTRACT
Gastric adenomyoma is a rare benign tumor composed of epithelial structures and smooth muscle stroma. Here, we report an unusual case of gastric adenomyoma mostly composed of smooth muscle that was incidentally found during a laparoscopic intervention. On radiology, it mimicked an acquired hypertrophic pyloric stenosis in an adult patient, and pathologically it resembled a pure smooth muscle hamartoma. Complete submission of the lesion for histology was necessary to find the epithelial component and make the right diagnosis. As a mimicker of benign and malignant entities, gastric adenomyoma is usually an unexpected finding after surgery. The aim of this report is to analyze this adenomyoma variant in the setting of an unexplained thickening of the gastric wall, with explanations concerning histogenesis and biological potential.
Keywords: Adenomyoma; Hamartoma; Pancreas/abnormalities;·Pyloric stenosis; Stomach neoplasms
RESUMO
O Adenomioma gástrico é um tumor benigno raro composto por estruturas epiteliais e por um estroma muscular liso. Aqui apresenta-se um caso invulgar composto maioritariamente por músculo liso, que foi identificado acidentalmente no decurso de uma intervenção laparoscópica. Na imagiologia a lesão simulava uma estenose pilórica hipertrófica adquirida do adulto e na anatomia patológica assemelhava-se a um hamartoma de músculo liso puro. A inclusão total da lesão para análise histológica permitiu encontrar o componente epitelial e o diagnóstico correto.Sendo um imitador de doenças benignas e malignas, o adenomioma gástrico é habitualmente um achado inesperado após a cirurgia. O objetivo da apresentação deste caso é reconhecer esta variante de adenomioma no contexto de um espessamento inexplicável da parede gástrica, com comentários sobre a sua histogénese e potencial biológico.
Palavras-Chave: Adenomioma; Hamartoma; Pâncreas/anomalias congénitas; Estenose pilórica; Neoplasias do estômago
Introduction
Gastric adenomyoma (GA) is a rare benign lesion composed of ducts and glands embedded in smooth muscle stroma. Several authors defined this entity as a hamartoma while others suggested that GA is best considered as an abortive variant of heterotopic pancreas (HP). In fact, some cases coexisted with HP and others also showed communication between the gastric lumen and the epithelial component of the lesion [1, 2] . Associated conditions may include annular pancreas, Gardner syndrome with duodenal adenomas, and gastric duplication [3] . According to Vandelli et al. [4] , 37 cases were recorded until 1993 and 15 cases were reported from 1993 to 2016, to the best of our knowledge. This entity affected patients aged from 1 month to 82 years, with a peak incidence between the fourth and sixth decades, without gender predilection [4, 5] . Several authors considered the gallbladder as the most frequent location of adenomyoma. Nevertheless, it is worth noting that gallbladder adenomyoma is defined as an exaggerated example of gallbladder diverticulosis [6] , composed of normal orthotopic tissues, and therefore, histogenetically different from gastrointestinal adenomyomas. Following other authors, the most common locations were the stomach (25–38%), duodenum (17– 36%), and jejunum (15–21%). In the stomach, GA was found in the antrum (85%), the pylorus (15%), and exceptionally in the body [7, 8] . Asymptomatic cases discovered incidentally during a laparoscopic intervention or autopsy are on record, although most of the reported cases presented with nonspecific symptoms such as nausea, vomiting, epigastric pain, hematemesis, anemia, and melena. Peritonitis secondary to perforation was also described [9] . Patients usually have a good clinical course after surgery. Depending on the size, location, and relative amount of tissue components, GA presented as a submucosal solid or cystic mass protruding into the gastric lumen [4, 8, 10] or as a localized thickening of gastric muscularis propria. The differential diagnosis includes gastric duplication, gastritis cystica profunda, and lymphoepithelial cyst [3] . When the adenomyoma is predominantly solid, gastrointestinal stromal tumors, leiomyoma, hamartoma, and a reactive hypertrophic smooth muscle condition must be considered. Imaging techniques are nondiagnostic for GA. In spite of the availability of newer diagnostic techniques, including endoultrasonography (EUS), it is still difficult to diagnose GA before operation [9, 10] . GA is considered a benign tumor, although a potential risk for malignant transformation exists [3, 11] .
Clinical Case
A 68-year-old woman presented with nausea and intermittent vomiting of 2 years duration. Ultrasound examination revealed the presence of cholelithiasis. Personal and familial history was irrelevant. During the programmed laparoscopic cholecystectomy, a white hard thickening of the anterior antral wall was found. After intervention, postprandial discomfort relapsed.
Serum tumor markers were negative. Abdominal computed tomography (CT) revealed a diffuse thickening of the antral wall without involvement of perigastric fat, enlarged lymph nodes, distant metastases, or pancreatic abnormalities ( Fig. 1 ). Endoscopic examination showed a slight prominence of antral mucosa without mass, ulcer, polyps, or stenosis. Endoscopic biopsy was inconclusive about the nature of the lesion, showing a moderate chronic gastritis. Helicobacter pylori (H. pylori) was absent. A reactive condition such as a developing acquired hypertrophic pyloric stenosis was the first hypothesis considered. The patient underwent a partial gastrectomy followed by Roux-en-Y reconstruction.
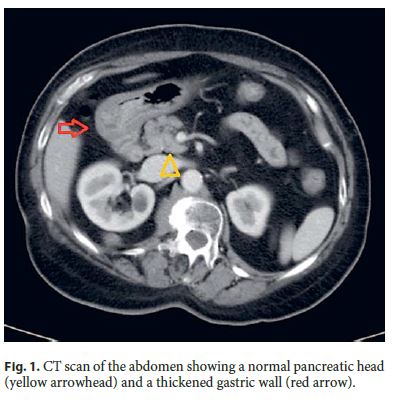
Surgical specimen was fixed in 4% buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. Immunohistochemical stainings were made by an indirect immunoperoxidase technique according to a standard protocol with antibodies against cytokeratin 7 (Dako clone OV-TL 12/30), CA 19.9 (Dako clone 1116-NS-19.9), smooth muscle actin (Dako clone 1A4), CD117 (Dako clone 104D2), CD34 (Dako clone QBEnd-10), S-100 protein (Dako clone IR504), and Ki 67 (Dako clone MIB-1).
The surgical specimen measured 7.2 cm along the greater curvature and the omentum measured 6.5 × 2 × 1 cm. Grossly, a diffuse thickening of the antral wall that measured 4 × 3 cm was identified, located 1 cm from the distal margin and 1.5 cm from the proximal margin. The maximum wall thickness was 0.9 cm. The submucosa showed areas of fat replacement and the overlying mucosa presented a slight superficial prominence of gastric folds ( Fig. 2 ). No lymph nodes were obtained. After a second look to the specimen, the lesion was completely submitted for histology.
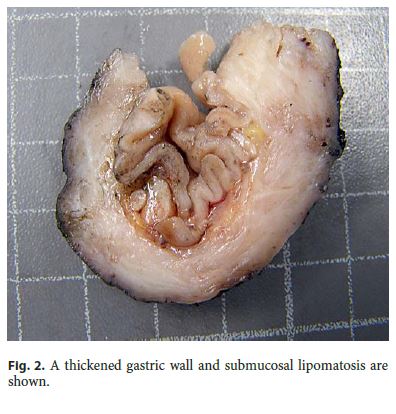
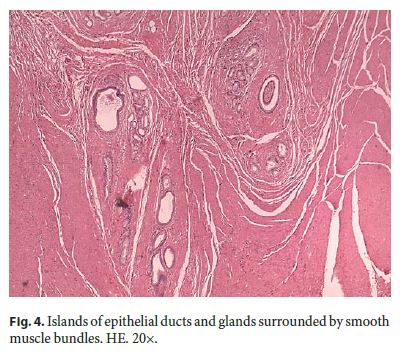
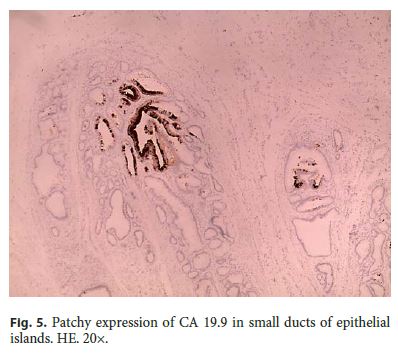
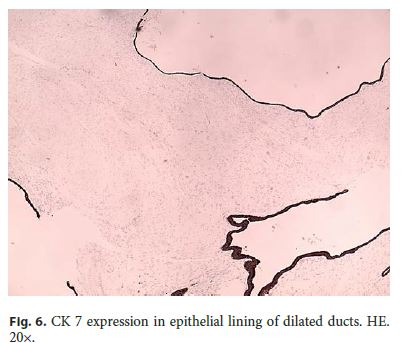
Microscopically, the first samples of the lesion revealed a thickened wall mostly composed of markedly disordered broad bundles of smooth muscle, and they were considered insufficient for diagnosis. After complete submission of the lesion for histology, a cribriform area of 1 × 0.5 cm was found (Fig. 3). It was composed of dilated ducts and islands of Brunner-type glands supported in scant loose connective tissue with sparse chronic inflammatory infiltrates (Fig. 4). Ducts and islands were embedded in disordered bundles of smooth muscle. Malignancy was not found, and the proliferation index observed with Ki67 was very low (<1%) in both muscular and epithelial components. Immunohistochemistry showed negativity for CD117, CD34, S-100, and consistent positivity for SMA in the smooth muscle component. Focal reactivity with CA 19.9 was observed in small ducts of epithelial islands but not in dilated ducts (Fig. 5). Expression of CK7 was uniform in epithelial lining of dilated ducts (Fig. 6). The adjacent submucosa showed patchy lipomatosis, loose fibrosis, and dilated vascular spaces. The overlying mucosa presented chronic gastritis with foci of incomplete pancreatic and intestinal metaplasia without dysplasia or malignancy. HP, defined as the presence of pancreatic tissue in the submucosa, muscular, or subserosa layers, was not identified. There was no continuity between gastric lumen and epithelial islands. H. pylori was absent in the mucosa and in the glandular component of adenomyoma. Surgical margins showed a normal gastric wall.
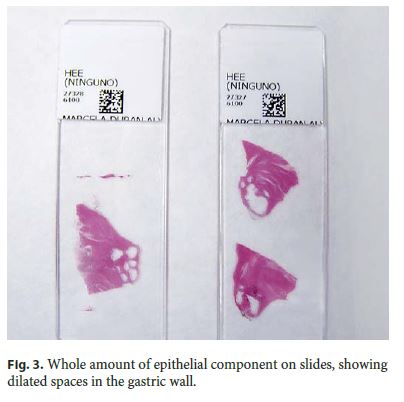
In addition, slides from previous cholecystectomy were reviewed and the diagnosis of chronic cholecystitis was confirmed.
Discussion
GA is a benign tumor composed of ducts, glandular structures, and smooth muscle stroma. This entity was first described in 1903 by Magnus-Alsleben. In the same year, Thorel noted the morphological similarity of GA and HP. The literature is rather confusing, with many reported cases using terms such as myoepithelial hamartoma, adenomyosis, adenomyomatous hamartoma or myoglandular hamartoma, and pancreatic heterotopia, reflecting disagreement on the histogenesis of this disease [1, 2, 11, 12] . Nevertheless, GA is widely accepted as developmental in origin [1–3, 5, 7–12] . The epithelial component is believed to originate from primordial epithelial buds which can differentiate into pancreatic or duodenal tissue. Smooth muscle component can accompany the embrionic epithelial buds or can originate from proliferative stimulation of mislocated epithelium [7, 11, 12] . These concepts can explain the presence of duodenal and/or pancreatic tissues in GA as reported in the literature. Moreover, the term hamartoma seems to be inaccurate for GA, since hamartoma defines a focal overgrowth of mature normal tissue in an organ of identical tissue elements. Several authors considered GA as an abortive variant of HP [2] . In 1909, Heinrich described 3 histological subtypes of HP and defined the third type as adenomyoma [13] . In our case, morphology and immunohistochemistry are consistent with a heterotopia composed of ambiguous, probably more primitive, epithelial phenotype. Another remarkable fact is the presence of pancreatic metaplasia in gastric mucosa in the absence of HP. This interesting finding, not previously described, raised the question about a possible biological commitment of some gastric epithelial cells to a pancreatic lineage in the setting of GA.
Submucosal lipomatosis and chronic gastritis were also interesting findings. The former, not previously described in GA, could be explained by chronic irritation secondary to mechanical forces acting over the slightly prominent mucosa and submucosa [14] .
In our case, the overgrowth of smooth muscle was the main source of mimic. We considered the likelihood of an acquired hypertrophic pyloric stenosis, but the disordered pattern of the lesion raised the suspicion for a hamartomatous lesion. Gastrointestinal stromal tumor was excluded on morphological and immunohistochemical analysis. Leiomyoma was also considered, but diffuse thickening of the muscularis propria is not a feature of gastric leiomyoma. Finally, pure smooth muscle gastric hamartoma is an extremely rare condition. The lack of consistency on the findings motivated complete submission of the lesion, which yielded the correct diagnosis.
Imaging techniques are nondiagnostic for GA. In this case, a preoperative CT scan was useful to exclude an advanced malignancy and also for surgical planning. Although magnetic resonance can identify the glandular component of the lesion, this finding is considered nonspecific [5] .
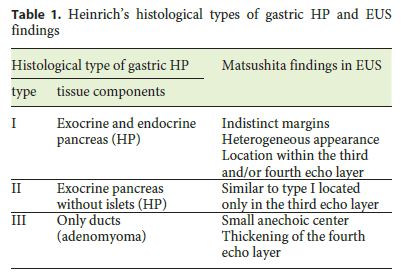
Endoscopic examination is also nondiagnostic, and endoscopic biopsy cannot provide representative samples from the deep layers of the gastric wall. In a few cases, GA presented as a polypoid mass feasible for endoscopic removal, although they were suspected to be hyperplastic polyps on endoscopy. Matsushita et al. [13] first characterized the EUS appearance of PH and GA following Heinrichs histological classification (Table 1), describing the latter as a small anechoic area (duct dilatation) and thickening of the fourth echo layer (muscular hypertrophy) [13] . Chu [15] also described the EUS characteristics of a case of a patient presenting as a submucosal nodule with a cystic center lined by a hyperechoic rim. Nevertheless, the diagnosis of GA remains exclusively histological based on the identification of heterotopic tissues, the architectural pattern of the lesion, its relation with the surrounding tissues, and the exclusion of malignancy. Therefore, the most important information determined by EUS is tumor location in the gastric wall and also the selection of good candidates for endoscopic removal of the lesion. In summary, since imaging techniques and endoscopic procedures are usually not sufficient to differentiate GA from other lesions, endoscopic or surgical resection is required to reliably diagnose an otherwise undefined gastric lesion.
Malignancy was not found in this case. We think that any misplaced tissue carries the same genetic pressure as any orthotopic tissue of the individual. What could make the difference is the environmental pressure of mislocation (heterotopia). In that sense, GA in continuity with gastric lumen may be colonized by H. pylori , as previously reported in the literature [10] . We consider that potential for malignant transformation exists and complete resection of the lesion is advisable. Our patient, as most patients reported in the literature, recovered successfully from the surgery, and she was free of symptoms on her first consultation after the intervention. In conclusion, complete submission of the lesion for histology provided new insights and findings on this entity. As a clinical and pathological mimicker of benign and malignant conditions, the incidence of GA in the general population could be higher than expected. To avoid misdiagnosis, thorough sampling of surgical specimen is recommended in the setting of an unexplained thickening of the gastric wall.
References
1 Janota I, Smith PG: Adenomyoma in the pylorus. Gut 1966; 7: 194–199. [ Links ]
2 Erberich H, Handt S, Mittermayer C, Tietze L: Simultaneous appearance of an adenomyoma and pancreatic heterotopia of the stomach. Virchows Arch 2000; 436: 172–174. [ Links ]
3 Ly DP, Barnard NJ, Schwarz RE: Gastric adenomyoma: definitely benign or defiantly premalignant? Dig Dis Sci 2004; 49: 1930–1934. [ Links ]
4 Vandelli A, Cariani G, Bonora G, Padovani F, Saragoni L, DellAmore D: Adenomyoma of the stomach. Surg Endosc 1993; 7: 185–187. [ Links ]
5 Sanchez Garcia S, Rubio Solis D, Anes Gonzalez G, Gonzalez Sanchez S: Gastric adenomyoma clinically simulating hypertrophic pyloric stenosis. Radiologia 2016; 58: 148–151. [ Links ]
6 Rosai J: Gallbladder and extrahepatic bile ducts; in Rosai J (ed): Rosai-Ackermans Surgical Pathology, ed 10. Philadelphia, Mosby-Elsevier, 2011, p 987. [ Links ]
7 Suma MT, Jakalatshmy PS, Resna R, Augustine J: Gastric adenomyoma causing outlet obstruction. OGH Reports 2016; 5: 45–47. [ Links ]
8 Yoon KH, Eun DY, Kim JH, Lee SO, Kim HS, Lee DW: Gastric adenomyoma in the stomach body: a case report. J Med Case Rep 2014;8: 385–388. [ Links ]
9 Zhu HN, Yu JP, Luo J, Jiang YH, Li JQ, Sun WY: Gastric adenomyoma presenting as melena: a case report and literature review. World J Gastroenterol 2010; 16: 1934–1936. [ Links ]
10 Lee H, Jang YJ, Heo J: A rare case of cystic subepithelial tumor in the stomach: gastric adenomyoma. J Korean Soc Radiol 2015; 73: 389–392. [ Links ]
11 Chapple CR, Muller S, Newman J: Gastric adenocarcinoma associated with adenomyoma of the stomach. Postgrad Med J 1988; 64: 801–803. [ Links ]
12 Clarke BE: Myoep ithelial hamartoma at thegastrointestinal tract. A report of eight cases with comment concerning genesis and nomenclature. Arch Pathol 1940; 30: 143–152. [ Links ]
13 Matsushita M, Takakuwa H, Nishio A: Endosonographic features of gastric adenomyoma, a type of ectopic pancreas. Endoscopy 2003;35: 621–622. [ Links ]
14 Jeong IH, Maeng YH: Gastric lipomatosis. J Gastric Cancer 2010; 10: 254–258. [ Links ]
15 Chu KM: Endosonographic appearance of gastric adenomyoma. Endoscopy 2002; 34:682. [ Links ]
Statement of Ethics
The authors declare that no experiments on humans or animals were performed in this study. The authors declare that they have followed the protocols of their work center on the publication of patient data. The authors have obtained the written informed consent of the patient.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Marcela Adriana Duran Álvarez
Department of Pathology, Medina del Campo Hospital
Carretera de Peñaranda, s/n
ES–47400 Valladolid (Spain)
E-Mail marduran12@yahoo.es
Received: August 29, 2016; Accepted after revision: November 7, 2016














