Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.6 Lisboa dez. 2017
https://doi.org/10.1159/000455180
CLINICAL CASE STUDY
Hodgkins Lymphoma in Crohns Disease Treated with Infliximab
Linfoma de Hodgkin em Doença de Crohn Medicada com Infliximab
Diana Carvalhoa, Pedro Russoa, Carlos Bernardesa, Joana Saiotea, Gonçalo Ramosa, Luis Mascarenhasb, Nuno Borgesc, Jaime Ramosa
aGastroenterology and Hepatology Department, bPathologic Department, and cGeneral Surgery Department, Centro Hospitalar de Lisboa Central, Lisbon, Portugal
* Corresponding author.
ABSTRACT
Introduction: Lymphoproliferative disorders, particularly non-Hodgkins and Hodgkins lymphomas, are rare in patients with inflammatory bowel diseases. The use of thiopurines and infection by Epstein-Barr virus are well-known cofactors that can raise its prevalence. Other risk factors such as disease activity and biological treatment are the subject of discussion, without enough data in the literature to confirm a potential association. Methods: We report a case of Hodgkins lymphoma in a patient who had been treated with azathioprine and was on long-term monotherapy with infliximab. Conclusions: We stress the importance of recognizing the possible occurrence of a lymphoproliferative disorder in association with anti-tumor necrosis factor-α therapy.
Keywords: Crohns disease, Hodgkins disease, chemically induced, Infliximab, Lymphoproliferative disorders, Tumor necrosis factor-α
RESUMO
Introdução: As doenças linfoproliferativas, em particular os linfomas não-Hodgkin e Hodgkin, são raras em doentes com doença inflamatória intestinal. O uso de tiopurinas e infecção pelo vírus Epstein-Barr são reconhecidos cofactores que podem aumentar a sua incidência. Outros factores de risco como a actividade da doença e o tratamento biológico são tema de discussão, não existindo dados suficientes na literatura para confirmar uma potencial associação. Métodos: Os autores descrevem um caso de linfoma Hodgkin num doente previamente medicado com azatioprina e em monoterapia de longa duração com infliximab. Conclusões: Este caso destaca a importância de reconhecer a possível ocorrência e/ou associação das doenças linfoproliferativas com a terapêutica com antifactor de necrose tumoral-α.
Palavras-Chave: Doenca de Hodgkin/induzida quimicamente, Doença de Crohn, Infliximab, Factor de necrose tumoral-α, Transtornos linfoproliferativos
Introduction
The search for an early and sustained mucosal healing, the current goal in the treatment of inflammatory bowel disease (IBD), led to an increased use of immunomodulators. However, this new paradigm is associated with the risk of adverse events, including the emergence of lymphoproliferative disorders [1]. Many processes are involved in this problem, including the direct immunosuppressive oncogenic effect of the drugs, the decreased immunological surveillance of mutant cells, and the impaired immune control of chronic infection by oncogenic microorganisms such as Epstein-Barr virus (EBV) [2].
In this setting, lymphomas are the most common lymphoproliferative disorder, especially non-Hodgkins lymphomas. Their incidence is slightly high in some population groups and can be associated with severe prognosis [3]. Hodgkins lymphomas (HL) are rare with a few numbers described in the literature [2, 4–6].
We present a case of HL in a patient with Crohns disease (CD) under treatment with infliximab (IFX) and previously treated with azathioprine.
Case Report
A 40-year-old man with ileocolonic non-stenotic, non-penetrating CD since 2000 was treated with azathioprine and IFX for steroid dependence in 2004. Afterwards, he underwent clinical and endoscopic steroid-free remission, although all immunosuppressive therapy was suspended in 2005, after he developed severe pneumonia.
Due to iron deficiency anemia that was poorly responsive to supplementation therapy and radiologic signs of CD activity, IFX was restarted 1 year later at 300 mg (5 mg/kg) every 8 weeks, followed by normalization of red blood cell count.
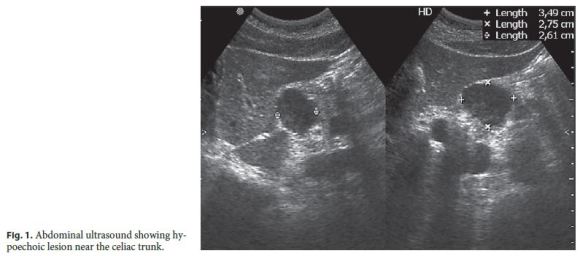
In September of 2012, after 6 years in remission under IFX monotherapy, a routine abdominal ultrasound revealed a hypoechoic oval lesion with defined contours, 3.5 cm in diameter, located at the fork of the celiac trunk, extrinsic to the liver and pancreas (Fig. 1), consistent with a lymphadenopathy on computed tomography (CT). Once it was diagnosed as a single adenopathy with no characteristics suggesting malignancy, a watch-and-wait strategy was adopted.
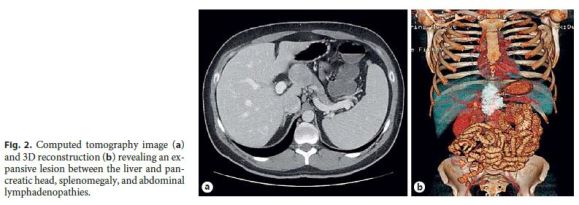
The patient remained asymptomatic, and 1 year later, a follow-up CT showed an oval lesion between the liver and the pancreatic head, with 40. 75 mm, as well as splenomegaly, portal vein enlargement, and multiple enlarged retroperitoneal lymph nodes. There were no radiological signs of active IBD (Fig. 2).
Complete blood count, C-reactive protein, protein electrophoresis, and immunoglobulin levels were normal. Viral serological tests, including EBV (VCA IgM, VCA IgG, and EBNA), were negative. Clinical and endoscopic remission was confirmed and values of calprotectin were negative. IFX was suspended.
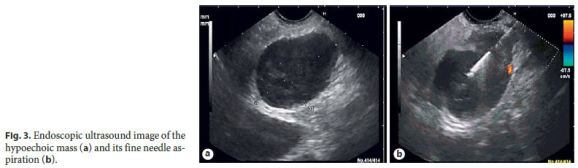
Endoscopic ultrasound revealed a heterogeneous hypoechoic mass, with well-defined limits, adjacent to the pancreatic isthmus and portal confluence. Fine needle aspiration was performed (Fig. 3) and the cytological study showed lymphoid CD20+ population, some macrophages, giant cells, and granulomas.
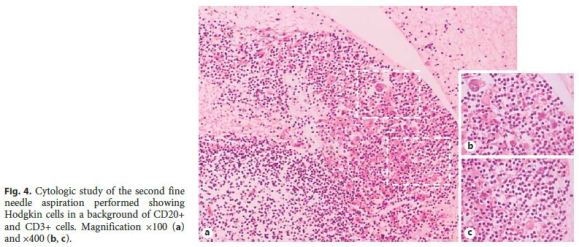
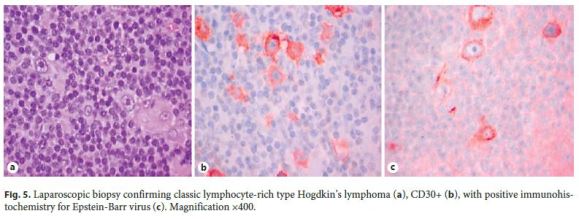
As these findings were suggestive, but nondiagnostic, of lowgrade B-cell lymphoproliferative disorder, a new endoscopic ultrasound with fine needle aspiration was carried out. Histology revealed a small number of nonnecrotizing granulomas and some large cells, with obvious nucleolus, of Hodgkins type, and intense staining for CD30 and CD15 in slight markup background of cells CD20+ and CD3+ (Fig. 4), all in favor of the diagnosis of HL. Flow cytometry did not show monoclonality. To confirm HL, a laparoscopic biopsy was performed, ultimately establishing the diagnosis of classic HL, lymphocyte-rich type, with positive immunohistochemistry for EBV (Fig. 5).
The immunological panel for EBV was repeated 3 months after the first one, where VCA IgG and EBNA IgG were positive; serum C-reactive protein for EBV was also positive, with a viral load of 1,990 copies. In the absence of new symptomatology, this apparent incongruence could be explained by a false negative initial test (e.g., laboratorial error) or eventually the prozone effect, which is rare in the case of EBV.
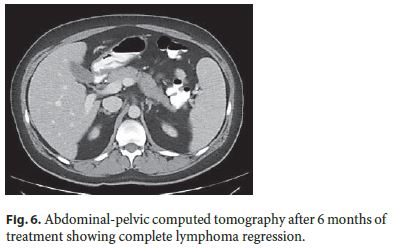
Stanford V protocol (cyclophosphamide, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, and prednisone, during 12 weeks) for the treatment of HL was initiated. Six months later, complete regression of abdominal lesions was confirmed by CT (Fig. 6). Presently, the patient remains with CD in remission and without signs of lymphoproliferative disorder.
At this point, with the patient being in remission, it was decided not to perform any treatment and maintain clinical and analytic (including calprotectin) monitoring. In case of recurrence, the treatment will be chosen based on the gravity and extension of the disease, taking into account therapy contraindications, especially thiopurines, in this setting.
Discussion
Lymphomas including HL are a known rare comorbidity that can emerge in IBD patients. Multiple risk factors are described including male gender, advanced age, genetic mutations, use of immunosuppressive therapy, and microorganisms such as EBV, human herpes virus 8, human T-lymphotropic virus 1, hepatitis C virus, and Helicobacter pylori [3].
There is not enough data to state that its incidence is raised in IBD. A recent population-based study including 2,325 IBD patients showed only an association between lymphomas and CD (SIR 3.01, 95% CI, 1.21–6.19), especially with non-Hodgkins lymphoma, regardless of theuse of thiopurines [7]. On the other hand, Chiorean et al. [8] in a population of 3,585 IBD patients (CD in 2,277 and ulcerative colitis in 1,308), with a total of 30,121 patient/years follow-up, did not find a significantly increased risk. A meta-analysis conducted by Pedersen et al. [9] revealed a tendency for a higher risk of lymphoma in patients with CD (SIR 1.42, 95% CI, 0.95–2.12).
The latest ECCO guidelines on this subject try to bring a consensus, stating that patients with IBD have a risk of developing hematological malignancies and CD patients are more likely to be at risk of lymphoma, in particular non-Hodgkins lymphoma [10]. However, it is difficult to distinguish the role of inflammation and immunomodulators in IBD-associated haematological diseases [1].
In fact, inflammation itself plays a role in lymphomagenesis in other inflammatory and autoimmune settings. Local sites of inflammation may be particularly at risk of lymphoproliferation, due to chronic B-cell stimulation [11]. In the French nationwide CESAME study cohort, IBD-associated lymphoproliferative disorders involving the gastrointestinal tract represent 26% of cases and the duration of the disease appears as an independent risk factor for lymphoma [12].
The association between immunosuppressive therapy and lymphomas is amply described in the literature. Thiopurines, the most frequent immunosuppressants used in IBD, can lead to diminished activity of DNA repair mechanism which favors a higher number of mutations. Furthermore, the clonal expansion is unregulated, leading to the emergence of lymphoproliferative disorders [2]. In CESAME, one of the biggest studies until today, the risk of lymphomas, including HL, was 5 times higher in patients under thiopurines compared to those who were never treated with these drugs [12]. This excess risk is present since the initiation of immunosuppressive therapy and remains until its cessation [11]. The authors found no difference between the group that stopped thiopurines and those who had never been treated with them [12].
Lymphomas have also been reported in patients treated with anti-tumor necrosis factor-α (TNF-α) drugs, which could act by decreasing apoptosis and allowing proliferation of neoplastic cells [2], but in most studies, anti-TNF-α appears to have no correlation with lymphomas [10]. In a register-based cohort study conducted by Nyboe Andersen et al. [13], 56,146 patients were included and 8 cases of lymphomas, with 2 HL, were diagnosed. There was a risk of 0.90 (95% CI, 0.42–1.91) for those who were under anti-TNF-α after adjusting the variable to the azathioprine use [13]. Then, caution must be taken, when associating lymphomas with anti-TNF-α therapy. In reported series, most of the patients were in combined treatment with thiopurines, which are a proven risk factor [3]. On the other hand, there are case reports in the literature showing partial or complete regression of HL after suspension of anti-TNF-α therapy, either for IFX or adalimumab, suggesting a role for these drugs [4, 5].
As for the concomitant therapy of anti-TNF-α and thiopurines, the absolute risk of lymphoma is not increased compared to the use of thiopurines alone, except for the case of hepatosplenic T-cell lymphoma, where more than 50% of patients had combined treatment [3]. However, these results must also be analyzed with caution because in most series, the number of patients is too low to detect significant differences [10].
EBV infection is a well-established risk for IBD-associated lymphoproliferative disorders. This virus from the herpes family is associated with the development of HL, especially in mixed cellularity subtype and in male patients [14]. It is responsible for 30–40% of HL in immunocompetent and 80% in immunocompromised patients. The association is clear when significantly higher levels of immunoglobulin G antibody anti-EBV (VCA, EA, and EBNA) are found in patients with HL compared to disease-free controls. Also the relation between EBNA 1 (needed for persistence of latent viral infection) and EBNA 2 (responsible for transformation of EBV-infected lymphocytes) is altered in these patients, with an EBNA 1:EBNA 2 ratio below 1 [15]. EBV can provide an antiapoptotic function, immortalizing the infected B cells [14]. Additionally, thiopurines destroy the natural killer and cytotoxic T cells responsible for the restriction of EBV proliferation; this lack in immunological surveillance allows the emergence of EBV oncogenic effect [12] increasing the risk of lymphoma.
Our patient had 4 risk factors for lymphoproliferative disorders: male gender, CD and its duration, and EBV infection. Treatment with azathioprine was stopped 7 years before the presentation of HL, so an association with this drug was unlikely. IFX may have contributed to the development and accelerated growth of HL, but its use for 12 months after the radiological detection of HL did not result in a worse outcome.
Currently, there is no algorithm for the diagnosis of lymphoproliferative disorders in patients with IBD treated with immunosuppressors and anti-TNF-α. The serological markers for EBV must be performed before choosing the therapeutic regime. Combination therapy must be avoided in young male patients to prevent the emergence of hepatosplenic lymphoma. In EBV-seronegative patients, thiopurines must be avoided and anti-TNF-α monotherapy should be preferred, thus lowering the risk of post-mononucleosis lymphomas [10]. Monitoring EBV viral load is recommended in posttransplant settings, but this approach has not been validated for patients with IBD, as a transitory increase can happen during flares [3]. In patients with headache, fever, enlarged lymph nodes, hepatomegaly, or splenomegaly, the possibility of a lymphoproliferative disorder should be considered [10]. In these patients, a diagnostic workup should be done and immunosuppressive therapy should be suspended.
In summary, HL can emerge in IBD patients on immunosuppressive therapy and physicians must be aware of this possibility. EBV plays a major role in its development, and routine EBV serological tests should be done to plan the better approach to the individual patient. Further studies are necessary to identify risk markers in IBD patients and construct an effective prevention algorithm.
References
1 Sifuentes H, Kane S: Monitoring for extra-intestinal cancers in IBD. Curr Gastroenterol Rep 2015;17:42. [ Links ]
2 Rodriguez AA, Kerner J, Luna-Fineman S, Berry GJ: Hodgkin lymphoma following adalimumab for the treatment of Crohns disease in an adolescent. Dig Dis Sci 2014;59:2403–2405. [ Links ]
3 Magro F, Peyrin-Biroulet L, Sokol H, Aldeger X, Costa A, Higgins PD, et al: Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis 2014;8:31–44. [ Links ]
4 Cassaday RD, Malik JT, Chang JE: Regression of Hodgkin lymphoma after discontinuation of a tumor necrosis factor inhibitor for Crohns disease: a case report and review of the literature. Clin Lymphoma Myeloma Leuk 2011; 11:289–292. [ Links ]
5 Mo N, Muthu S, OSullivan M: Regression of lymphoma after withdrawal of infliximab alone in an infliximab/methotrexate-treated RA patient. Rheumatology 2011;50:808–810. [ Links ]
6 Dahhan T, Al Kahtani K, Bakshi N, Abouzied ME, Helmy A: Extra-intestinal Hodgkins lymphoma in a Crohns disease patient on long-term azathioprine and infliximab therapy. Trop Gastroenterol 2010;31:51–53. [ Links ]
7 Jess T, Horvath-Puho E, Fallingborg J, Rasmussen HH, Jacobsen BA: Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol 2013;108:1869–1876. [ Links ]
8 Chiorean MV, Pokhrel B, Adabala J, Helper DJ, Johnson CS, Juliar B: Incidence and risk factors for lymphoma in a single-center inflammatory bowel disease population. Dig Dis Sci 2011;56:1489–1495. [ Links ]
9 Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T: Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010;105:1480–1487. [ Links ]
10 Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, et al: European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis 2015; 945–965. [ Links ]
11 Sokol H, Laurent B: Lymphoma risks in IBD. In: Bayless TM, Hanauer S. editors. Advanced Therapy in Inflammatory Bowel Disease. Volume I: IBD and Ulcerative Colitis. 3rd ed. Shelton: Peoples Medical Publishing House-USA; 2011, pp 489–497. [ Links ]
12 Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, et al: Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–1625. [ Links ]
13 Nyboe Andersen N, Pasternak B, Basit S, Andersson M, Svanstrom H, Caspersen S, et al: Association between tumor necrosis factoralpha antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA 2014;311:2406–2413. [ Links ]
14 Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, et al: The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol 2015;235:312–322. [ Links ]
15 Coghill AE, Hildesheim A: Epstein-Barr virus antibodies and the risk of associated malignancies: review of the literature. Am J Epidemiol 2014;180:687–695. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
Doctor Jaime Ramos has been a consultant for MSD and Hospira and received fees for conferences from Abbvie. The other authors declare to have no competing interests.
* Corresponding author.
Dr. Diana Carvalho
Department of Gastroenterology and Hepatology
Hospital Santo Antonio dos Capuchos, Centro Hospitalar de Lisboa Central
Alameda Santo Antonio dos Capuchos, PT–1169-050 Lisbon (Portugal)
E-Mail dianafbcarvalho@gmail.com
Received: September 12, 2016; Accepted after revision: December 15, 2016














