Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.6 Lisboa dez. 2017
https://doi.org/10.1159/000461593
CLINICAL CASE STUDY
Adult Celiac Disease: The Importance of Extraintestinal Manifestations
Doença celíaca do adulto: a importância das manifestações extraintestinais
Gonçalo Nunesa, Rita Barosaa, Marta Patitaa, Vítor Fernandesa, Diogo Gonçalvesb, Jorge Fonsecaa–c
Departments of aGastroenterology and bPathology, Hospital Garcia de Orta, Almada, and cCiiEM, Centro de Investigação Interdisciplinar Egas Moniz, Monte da Caparica, Portugal
* Corresponding author.
ABSTRACT
Celiac disease (CD) is a chronic immune-mediated enteropathy driven by gluten and affecting individuals of all ages. The diagnosis of CD in adulthood is emerging and patients often present with nonclassical extraintestinal manifestations. We report the case of a 53-year-old man presenting with neuromuscular symptoms, skin rash, inconspicuous chronic diarrhea, marked weight loss, and biochemical markers of malabsorption. A strong clinical suspicion led to the diagnosis of CD with clinical recovery after the initiation of a gluten-free diet. Clinical presentation with atypical symptoms in adult CD patients is the rule and not the exception. Most of the extraintestinal manifestations depend on background autoimmune phenomena and micronutrient malabsorption. A gluten-free diet re-establishes homeostasis and prevents long-term complications.
Keywords: Celiac disease, Gluten, Extraintestinal manifestations
RESUMO
A Doença celíaca (DC) é uma enteropatia crónica imunomediada precipitada pela ingestão de glúten, afetando indivíduos de todas as faixas etárias. O diagnóstico de DC está a emergir, com os doentes a apresentarem-se frequentemente com manifestações extraintestinais não clássicas. Os autores descrevem o caso de um homem de 53 anos com sintomas neuromusculares, rash cutâneo, diarreia crónica não valorizada, marcada perda ponderal e evidência bioquímica e laboratorial de má absorção. O elevado nível de suspeição clínica conduziu ao diagnóstico de DC com recuperação clínica total após instituição de dieta sem glúten. A apresentação clínica da DC na idade adulta com sintomas atípicos é a regra e não a exceção. A maioria das manifestações extraintestinais está dependente de fenómenos de autoimunidade e má absorção de micronutrientes. A dieta sem glúten permite reestabelecer a homeostasia e prevenir complicações a longo prazo.
Palavras-Chave: Doença celíaca, Glúten, Manifestações extraintestinais
Introduction
Celiac disease (CD) is a chronic immune-mediated form of enteropathy affecting the gut in genetically predisposed patients. It is driven by the ingestion of food containing gluten, a protein conglomerate whose main component is gliadin, present in wheat, rye, barley, and processed foods [1–5]. CD must be distinguished from wheat allergy and nonceliac gluten sensitivity that may have a similar clinical presentation [1, 2].
In the past, CD was considered a pediatric disease, mostly diagnosed in children who presented with severe malnutrition, chronic bulky diarrhea, and failure to thrive. In fact, the prevalence of CD in the adult population ranges between 0.3 and 1% [1]. On average, adult CD is diagnosed at around 45 years of age in adulthood, although 25% of patients present above the age of 60 years [1]. There is a clear genetic predisposition related with the human leukocyte antigen (HLA) haplotype polymorphisms, namely HLA DQ2 and HLA DQ8 [1].
The diagnosis is easy when CD presents with typical features of malabsorption, a positive celiac serology, and duodenal villous atrophy (classic CD). However, in most adult patients, gastrointestinal symptoms are subtle or even absent and clinical suspicion should be oriented to the extraintestinal manifestations (nonclassic or atypical CD), including anemia, skin disorders, neuromuscular symptoms, bone disease, and abnormal liver function tests. A life-long gluten-free diet is mandatory, achieving clinical and histological recovery in most patients [3].
The present report describes a case of CD in an adult patient with several extraintestinal manifestations. We aim to highlight the importance of considering nontypical symptoms to diagnose CD in adulthood and enforce an early gluten free-diet in order to control clinical symptoms and prevent long-term complications.
Case Report
A 53-year-old Caucasian man, living in Angola, sought for medical attention due to generalized myalgia and nocturnal episodes of lower limb paresthesia and muscle cramps since 3 months. At the same period, he developed abdominal pain and diarrhea with 3–6 loose, bright, and greasy stools per day suggestive of steatorrhea, marked weight loss (10 kg corresponding to 14% of his body weight), and a facial skin rash characterized by itchy papulae and vesicle lesions.
His past medical history included malaria and typhoid fever, with no other previous diseases. The patient worked as a construction worker, did not maintain direct contact with animals, and did not drink potential contaminated water. Alcohol consumption was 40 g per day with no smoke, toxic, and other xenobiotic exposure. He did not take any medication. The family history was unremarkable.
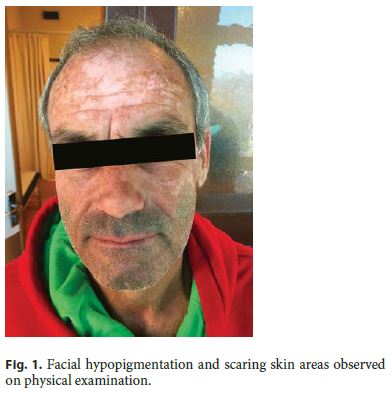
The initial tests carried out by the general practitioner revealed negative HIV serology, stool culture, and Clostridium difficile toxin. The ileocolonoscopy was normal, including endoscopic biopsies from normal appearing mucosa. Abdominal CT scan did not show any sign of chronic pancreatitis or intra-abdominal cancer. The patient was referred to the outpatient gastroenterology clinic of our hospital 4 months after the beginning of his symptoms. Physical examination was remarkable for malnutrition (body mass index 19), with loss of muscle mass and hypopigmentation and scaring skin areas in the face (Fig. 1) .
Laboratory assessment in our hospital revealed macrocytic anemia (hemoglobin 11.4 g/L; mean corpuscular volume 100 fL), ferritin 148 ng/mL, low folate (2.2 ng/mL), normal vitamin B12 and ferritin levels, abnormal clotting tests (prothrombin time 19 s; partially activated thromboplastin time 37.2 s), hypokalemia (3.2 mmol/L), low total serum proteins (6.3 g/L), and a normal albumin concentration (3.5 g/dL). Gamma globulins, liver biochemistry, creatinine phosphokinase, and thyroid hormones were normal. IgA anti-tissue transglutaminase antibodies (IgA-tTG) were positive.
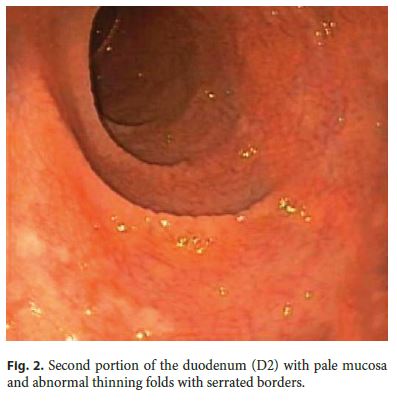
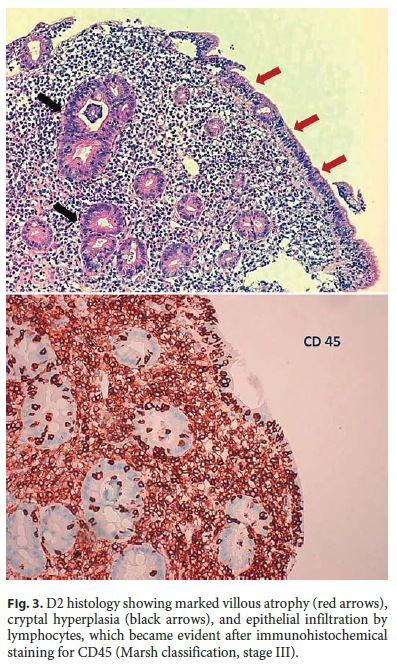
Upper gastrointestinal endoscopy showed abnormal duodenal morphology characterized by a smooth tubular surface, pale mucosa, and abnormal thinning folds with serrated borders (Fig. 2) . Biopsies were performed and histology was suggestive of CD with marked villous atrophy, cryptal hyperplasia, and epithelial infiltration by lymphocytes (Marsh classification, stage III) (Fig. 3).
A gluten-free diet was enforced and after 2 months, the patient was completely asymptomatic without diarrhea and neuromuscular symptoms. He recovered 4 kg and his skin lesions improved.
Discussion
In this report, we described a case of CD diagnosed in a patient over 50 years of age. Although the patient presented some gastrointestinal symptoms, he sought and attracted medical attention due to several extraintestinal disorders, namely neuromuscular symptoms and cutaneous rash.
CD is thought to be underdiagnosed in adulthood in part owing to the fact that physicians are not aware of the high prevalence of extraintestinal manifestations in this age group. In addition, many patients do not have diarrhea nor classical signs of malnutrition [1, 3]. Thus, doctors must be aware of CD presentation with atypical symptoms since many patients may seek medical advice because of them, as it was the case in our patient.
The pathophysiology of extraintestinal manifestations is not fully understood. Some of these manifestations are a direct consequence of autoimmunity, like dermatitis herpetiformis, gluten ataxia, and central nervous system demyelination lesions, whereas others are indirectly related to inflammation and/or malabsorption, including anemia, osteoporosis, infertility, and certain neuropsychiatric symptoms. In our patient, malabsorption clearly prevails [4]. He presented with a skin rash with an initial description of itchy papulae and vesicle lesions suggestive of herpetiformis dermatitis. However, when the patient came to our institution, the lesions were quite different showing an unspecific scarring pattern. Our patient presented also with myalgia and lower limb paresthesia and muscle cramps which probably could be attributed to a peripheral polyneuropathy. We admit that dermatitis and polyneuropathy might be associated with some micronutrient deficiency that are not routinely measured in clinical practice such as vitamin A, complex B vitamin, or zinc deficiency. Of note, vitamin B12 levels were normal in our patient who had also anemia and abnormal clotting times probably due to folate and vitamin K malabsorption, respectively. This is suggestive of duodenal and jejunal malabsorption with preserved ileal function. Hypokalemia was probably associated with gastrointestinal losses despite other electrolytes, such as calcium, phosphorus, and magnesium, are normal. Low calcium levels may be observed in CD patients due to malabsorption, which could be associated with the development of bone disease. An increased prevalence of osteoporosis may justify the request of an osteodensitometry and calcium plus vitamin D supplementation in the follow-up of this patient.
CD screening is usually accomplished using serology, but in most cases, a definite diagnosis must be confirmed with a duodenal biopsy, as performed in our patient. The exception to this rule is in patients with a biopsy proven herpetiformis dermatitis [1]. IgA anti-tissue transglutaminase are the most sensitive and cost-effective antibodies to diagnose CD, despite deamidated gliadin peptide IgG antibodies might be useful in seronegative patients with innate IgA deficiency [1]. In face of a strong clinical suspicion even when antibodies are negative, only a duodenal biopsy can confirm the diagnosis. Nevertheless, it should be considered that other conditions can mimic duodenal changes usually observed in CD, including tropical sprue, bacterial overgrowth, and Whipple disease. HLA DQ2 and DQ8 sequencing may be helpful in this context, given their high negative predictive value [1].
A gluten-free diet is mandatory with 70% of patients improving after 2 weeks of nutritional therapy [1]. All patients should have dietetic advice at diagnosis. Control biopsy is not routinely recommended, since several studies have shown a clear association between clinical remission and mucosal recovery [5]. Serology allows monitoring patient adherence to therapy, and antibodies are expected to become negative few months after gluten withdrawal [5]. After 2 months of a gluten-free diet, our patient became completely asymptomatic confirming the effectiveness of this approach. Nevertheless, a small group of patients, circa 5%, have refractory CD and do not respond after 6–12 months of dietary gluten avoidance. This condition is more common when CD is diagnosed in adulthood and is associated with a poor prognosis [1, 5].
CD was diagnosed in our patient after 50 years of age, with his symptoms clearly beginning 4 months before the diagnosis. However, it is not clear if the patient had a previous latent CD which manifested in adulthood or if the disease developed de novo at this time. This case report highlighted the importance of considering extraintestinal manifestations in CD patients presenting in adulthood and the effectiveness of the introduction of a gluten-free diet to solve symptoms and to re-establish digestive physiology and homeostasis.
References
1 Bai JC, Fried M, Corazza GR, et al: World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol 2013;47:121–126. [ Links ]
2 Allen PJ: Gluten-related disorders: celiac disease, gluten allergy, non-celiac gluten sensitivity. Pediatr Nurs 2015;41:146–150. [ Links ]
3 Leffler DA, Green PH, Fasano A: Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol 2015; 2:561–571. [ Links ]
4 Hvas CL, Jensen MD, Reimer MC, et al: Celiac disease: diagnosis and treatment. Dan Med J 2015;62:C5051. [ Links ]
5 Green PH, Cellier C: Celiac disease. N Engl J Med 2007;357:1731–1743. [ Links ]
Statement of Ethics
All procedures performed were in accordance with the ethical standards of the institutional and/or national committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Disclosure Statement
The authors do not have any potential financial, professional, or personal conflicts regarding this study.
* Corresponding author.
Dr. Gonçalo Nunes
Department of Gastroenterology, Hospital Garcia de Orta
Av. Torrado da Silva
PT–2805–267 Almada (Portugal)
E-Mail goncalo.n@hotmail.com
Received: January 6, 2017; Accepted after revision: January 31, 2017














