Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.3 Lisboa jun. 2018
https://doi.org/10.1159/000481537
REVIEW ARTICLE
Endoscopic Retrograde Cholangiopancreatography and Endoscopic Ultrasound: To Be One Traveler in Converging Roads
CPRE e Ecoendoscopia: um mesmo viajante em estradas convergentes
Pedro Moutinho-Ribeiro, Armando Peixoto, Guilherme Macedo
Gastroenterology Department, Centro Hospitalar São João, and Porto World Gastroenterology Organization Training Center, University of Porto Medical School, Porto, Portugal
* Corresponding author.
ABSTRACT
Background: Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) were initially introduced into the world of gastroenterology as purely diagnostic procedures. With progressive evolution of intervention, both these techniques conquered fields in the treatment of many conditions that had once been exclusively surgical domains. Nowadays, more and more clinical situations have an indication to perform both EUS and ERCP, and these two techniques are frequently required at the same time for the same patient. More than competitors, ERCP and EUS are truly complementary, with great ability for mutual aid. They share their main indications, equipment, accessories, and main technical gestures. Objectives and Methods: We review the major indications to perform both techniques, sequentially or complementarily, describe the common things that these two techniques essentially share, and discuss the ERCP-EUS single session. Also, the issues of learning curves and education of upcoming biliopancreatic endoscopists are highlighted. Conclusion: In recent years the complementation between ECRP and EUS has been growing both from a diagnostic and a therapeutic point of view, allowing optimization of the use of these techniques and the creation of a more systematized approach of patients with biliopancreatic pathology. Endoscopists with experience in both techniques will be increasingly important, suggesting a parallel formation in the training plans of future endoscopists with interest in the area.
Keywords: Biliary drainage, Endoscopic retrograde cholangiopancreatography, Endoscopic ultrasound, Endoscopy, Single-session procedure, Therapeutics
RESUMO
Introdução: A colangiopancreatografia retrógrada endoscópica (CPRE) e a ultrassonografia endoscópica (EUS) foram introduzidas no mundo da Gastrenterologia há algumas décadas, inicialmente como procedimentos puramente diagnósticos. Com a evolução progressiva na área da intervenção, estas duas técnicas conquistaram terreno no tratamento de muitas patologias que, outrora, eram exclusivamente do domínio cirúrgico. Atualmente, cada vez mais situações clínicas têm indicação para a realização de EUS e CPRE e, frequentemente, ambas são solicitadas ao mesmo tempo para um mesmo doente, a realizar idealmente em sessão anestésica única. Mais do que concorrentes, a CPRE e a EUS são técnicas verdadeiramente complementares, com elevada capacidade de interajuda. Compartilham as suas indicações mais frequentes, equipamentos e acessórios, bem como os principais gestos técnicos. As duas técnicas têm, também, em comum curvas de aprendizagem bastante exigentes. Objetivos e métodos: O presente artigo tem como objetivo rever as principais indicações para a realização de EUS e CPRE, sequencialmente ou de forma complementar, descrever os aspetos comuns a ambas as técnicas e discutir o conceito de EUS-CPRE em sessão única. São ainda equacionados os desafios futuros, com especial foco nas questões relativas à formação dos futuros gastrenterologistas, de modo a promover o contínuo aperfeiçoamento da endoscopia biliopancreática, diagnóstica e terapêutica. Conclusão: Nos recentes anos a complementação entre a ECRP e a EUS tem vindo a crescer tanto do ponto de vista diagnóstico, como terapêutico, permitindo uma optimização da utilização destas técnicas e a criação de uma abordagem mais sistematizada dos doentes com patologia biliopancreática. Endoscopistas com experiência em ambas as técnicas serão cada vez mais importantes, sugerindo-se uma formação paralela nos planos de formação de futuros endoscopistas com interesse na área.
Palavras-Chave: Colangiopancreatografia retrógrada endoscópica, Ultrassonografia endoscópica, EUS-CPRE em sessão única, Patologia biliopancreática
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) was introduced in the world of gastroenterology four decades ago initially as a purely diagnostic procedure, but after the development of endoscopic sphincterotomy and increasingly complex techniques, it moved to the therapeutic area. Furthermore, the emergence and rapid improvement of less invasive imaging modalities, such as computed tomography, magnetic resonance cholangiopancreatography, and endoscopic ultrasound (EUS), replaced ERCP in its diagnostic role. In fact, this technique is mainly considered a therapeutic procedure. Despite this shift to intervention, the main indications for its performance have not changed, namely cholelithiasis and its complications, biliary and pancreatic ductal abnormalities, and ampullary/periampullary lesions [1, 2].
EUS was introduced in the 1980s, with the first scopes with mechanical technology and radial scan. As ERCP, EUS was restricted to a purely diagnostic technique at the beginning. In the early 1990s, after the appearance of the first linear scope, it became possible to perform EUS-guided fine needle aspiration (FNA). It also opened the gate to exploring the gut wall and the peridigestive organs and spaces, either for diagnostic or therapeutic purposes. The development of electronic transducers led to the ability to improve ultrasound imaging, allowing Doppler function, double harmonic and, more recently, elastography and echo contrasts. The evolution of the scopes lumen working channels and FNA needles caliber, namely the emergence of the 19G needle, has turned EUS into a modality that combines an important diagnostic accuracy, playing a role in locoregional staging of a wide range of malignancies, with an increasing number of therapeutic procedures [3]. These intervention techniques include drainage procedures of biliary and pancreatic ducts, peridigestive collections, interluminal anastomoses and, more recently, antitumor therapies and vascular interventions [3]. Much of this long and auspicious way was simplified by the fact that EUS procedures share numerous devices and accessories used and developed for ERCP [4].
What Do These Techniques Essentially Share?
Importantly, they are both advanced endoscopic techniques. Their history was common, starting in the diagnostic field with progressive evolution to intervention, conquering fields in the treatment of many conditions that had once been exclusively part of the surgical domain. Many of their associated procedures have in fact significant levels of invasiveness, with inevitable associated risks of adverse events and complications.
Above all, they also share one of their main indications: biliopancreatic pathology. In this field, they are two techniques that more than competitors; they are truly complementary, with great ability for mutual aid. Therefore, it is not surprising that more and more, in several clinical situations, they are being requested at the same time for the same patient, and the once called ERCP room facility should include standing equipment for therapeutic EUS.
There is thus a convergence of nosological interests and the capacity to contribute to their diagnosis and treatment, through the use of similar equipment (duodenoscope and linear echoendoscope) and common accessories and devices (such as guidewires, stents, and contrasts) that allow performance of complex maneuvers, of course with inherent risks of adverse events. In fact, the technical evolution of the scopes and devices ran parallel for both modalities, with improvements in each of them contributing to progresses in its counterpart.
The similitude of equipment, accessories, and main technical gestures is such that they both share the need of having a dedicated and experienced team, especially nurses with properly advanced training in endoscopy and anesthesiologists fully orientated to these patients, and specific procedures [5]. In both, outcomes after diagnostic and/or therapeutic endoscopy are influenced by endoscopists level of expertise and the centers case volume.
Learning Curves
As advanced techniques, their learning curves are steep and very demanding [6, 7]. The programs of education of learning ERCP and EUS, both in the internship and in the postgraduation period, should consider that for those really interested in interventional endoscopy, the next level for acquisition of skills should be concurrent and complementary in both techniques, specially focused on biliopancreatic diseases.
Endoscopic Retrograde Cholangiopancreatography
Implementing a standardized protocol for an ERCP training program is very difficult and might not be feasible in practice. Procedure volume, indications, and technical approaches vary widely among different countries, institutions, and even endoscopists in one institution. Competence has been classically evaluated according to a minimum number of procedures performed, and the number of procedures recommended in most training programs ranges from 100 to 200. In particular, the American Society for Gastrointestinal Endoscopy (ASGE) recommends a minimum of 180–200 ERCPs [8].
However, a more dedicated perspective of the quality of procedures is lacking, which is not necessarily the result of a greater number of procedures. Current research supports establishing a standard of 80–90% technical success before trainees are deemed competent in a specific skill [9]. Nevertheless, individual trainees may differ in the acquisition of technical skills. Therefore, these threshold numbers might be inadequate. Recently, a study reported the use of a standardized form for continuous self-assessment. The form included previously proposed quality indicators for ERCP such as procedural indication, degree of technical difficulty, previous ERCP failure, and success or failure options (such as cannulation of the common bile duct [CBD] or pancreatic duct, stent placement, sphincterotomy, and stone extraction) [9]. This method allowed not only for the quantitative evaluation of trainees, but also determined the learning curve of each individual and of the average group progression. As such, current training should probably be based on learning curves, although more studies are needed to validate these resources.
Endoscopic Ultrasound
The limited availability of EUS is largely the result of a lack of skilled endosonographers. A relative lack of training centers combined with the extensive commitment required by the trainee has limited the growth of EUS and its availability in community practice. For most train-ees, the amount of EUS exposure and training is highly variable and often program-dependent. Many fellowship programs do not provide the opportunity to learn EUS.
The ASGE recommends a minimum of 150 total supervised procedures, 75 of which should be pancreatobiliary and 50 FNA (25 of which pancreatic FNA) before competency can be determined [10]. The learning process of EUS-FNA has been studied for solid pancreatic lesions: a learning curve with increasing sensitivity in the cytopathological diagnosis of cancer (reaching 80% after 20–30 EUS-FNA), with a decreasing number of passes needed (median of 3 after 150 EUS-FNA). Also, trainees should demonstrate competence in linear EUS before undertaking EUS-FNA. In Europe, the European Society of Gastrointestinal Endoscopy (ESGE) endorses the use of a combination of different simulators and, if available, live pigs during training in EUS-FNA [11]. A minimum of 20–30 supervised nonpancreatic and pancreatic lesions, respectively, should be performed with rapid on-site cytopathological examination.
Common Indications
There is an increasing number of pathologies which benefit from a common EUS-ERCP approach. The most frequent one is biliary stone disease and its complications. In Table 1, the indications for the joint use of ERCP and EUS are summarized.
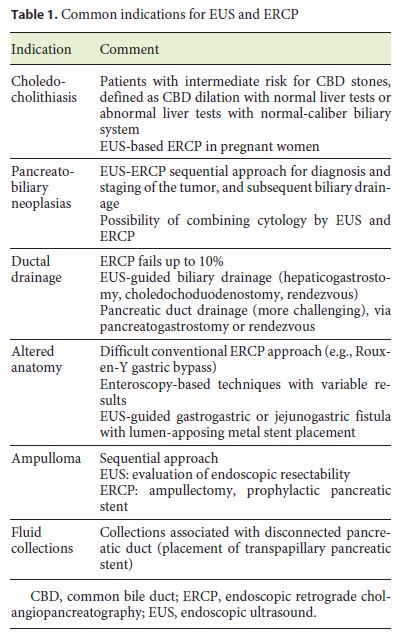
Choledocholithiasis
As it is undisputed that ERCP is the technique of choice for the treatment of choledocholithiasis, recent randomized trials have proven the benefit of performing a previous diagnostic examination in order to confirm the presence of calculi in the CBD, especially in subjects with intermediate risk for it (CBD dilation with normal liver tests or abnormal liver tests with normal-caliber biliary system) [12]. Among these techniques, magnetic resonance cholangiopancreatography and EUS are good alternatives, the latter being more accurate to detect small stones and being the option when MRI is contraindicated [13]. In fact, in two extensive meta-analyses using ERCP or surgical exploration as criterion standards, EUS revealed a sensitivity of 89–94% and a specificity of 94–95% in the evaluation of choledocholithiasis [14, 15]. It is estimated that EUS can be used in selecting patients for therapeutic ERCP, avoiding diagnostic ERCPs and their complications in 60–75% of cases [4, 16].
The EUS plus ERCP strategy is feasible, effective, and safe, with no increase in procedure- or sedation-related complications [17, 18]. This is particularly important in the case of elderly patients, where reports proved that long-time procedures were not related to more adverse events [19]. Another interesting group of patients are pregnant women. Vohra et al. [20] reported a successful single-session EUS-based ERCP in 10 pregnant patients with suspected choledocholithiasis. In this case series, EUS immediately prior to scheduled ERCP eliminated the need for ERCP and also its risks in pregnant wom-en with no evidence of choledocholithiasis on EUS. In patients with confirmed choledocholithiasis, EUS provided additional information regarding the location, number, and size of bile duct stones, which enabled the successful clearance of the bile duct without the use of fluoroscopy.
Knowing that cannulation of the papilla with a linear EUS scope is difficult and may be not possible in a great number of patients, it is very attractive to have a new EUS scope also designed for biliopancreatic cannulation. In 2006, Rocca et al. [21] reported the EUS diagnosis and simultaneous endoscopic retrograde cholangiography treatment of CBD stones by using an oblique-viewing echoendoscope in 19 patients with acute abdominal pain associated with increased liver tests. When biliary stones or sludge were found, bile duct cannulation and sphincterotomy were performed in the same session. In this study, this approach was feasible and safe, providing an accurate diagnosis and, at the same time, an appropriate treatment of CBD stones. Technical improvements are needed so that, in the near future, combined EUS-ERCP scopes can be used in the management of patients with biliopancreatic diseases.
Diagnosis of Pancreatobiliary Neoplasias
Another group of pathologies in which the roles of EUS and ERCP converge is the one related to biliary and pancreatic obstructions, particularly when a malignant cause is suspected, such as pancreatic head neoplasia. In these patients, an EUS-ERCP sequential approach has clear advantages, the former with an important role in thecorrect diagnosis (with the possibility of performing FNA) and staging of the tumor, and the latter being the technique of choice when biliary drainage is indicated. In these cases, the information provided by EUS in relation to tumor stage may contribute to the choice of drainage modality, namely the option of a plastic or metallic stent [4].
Concerning the diagnosis of biliary stricture itself, it is agreed that EUS-FNA has higher accuracy than ERCP cytology, especially in cases where there is a mass-forming lesion (sensitivity of 43–86%) [22–25]. In fact, albeit brush cytology has been the mainstay for suspected malignant biliary strictures because it is easy and safe to perform, the overall sensitivity for cancer is only 30–57% [26]. Addition of endobiliary forceps biopsy improves sensitivity, but it is time consuming, increases costs, and may not be technically feasible [27]. Nevertheless, when strictures are not associated with a mass-forming lesion, ERCP cytology with or without forceps biopsy seems to be superior to EUS-FNA [4]. Another chance for interventional EUS to come close to ERCP is the exponentially growing indications for interventional EUS in biliopancreatic drainage procedures.
Although it is the preferred technique, ERCP may fail or not be possible in up to 10% of cases (anatomical variants, previous surgeries, tumor extension, operator disability, etc.) [4, 28]. In this context, EUS is assumed as a first-line rescue option, with advantages in relation to surgery or percutaneous drainage. In fact, when compared to percutaneous drainage, EUS-guided drainage has a similar technical success rate, but fewer procedure-related adverse events and fewer unscheduled re-interventions [28].
Ductal Drainage Procedures
In 1996, Wiersema et al. [29] described the first EUS-guided puncture of the bile duct to perform a diagnostic cholangiography. Five years later, Giovannini et al. [30] reported the first EUS-guided biliary drainage procedure, a bilioduodenal anastomosis. Since then, many refinements in drainage techniques have been observed and, at present, EUS is indicated as a procedure of choice in several situations, either for biliary and/or pancreatic duct access, fluid collections and, more recently, gallbladder drainage.
Regarding biliary strictures, more than 1,000 cases have been published by now, with technical and clinical success rates of 91 and 88%, respectively. The mean overall complications (16–35%) and mortality rates (1–5%) are variable among series, being higher in the early stages of the operator learning curve [4], so these procedures should be reserved for tertiary centers with expertise in both EUS and therapeutic endoscopy. For EUS-guided biliary drainage, depending on the type and location of the lesion, the intra- or extrahepatic routes can be used to access the ductal system, with similar technical and clinical success rates, although the latter seems to be safer. After access, stent placement can be made directly (hepaticogastrostomy or choledochoduodenostomy), or through a rendezvous procedure with ERCP, if passing a transpapillary guidewire is achievable. A third alternative is placement of the stent in an anterograde way, through the papilla [31].
Pancreatic duct drainage guided by EUS is usually indicated in obstructions due to benign disorders, such as stenosis, lithiasis, or postoperative strictures. After main pancreatic duct access, usually through the stomach, the drainage procedure may equally consist of the placement of a stent transluminally (pancreatogastrostomy) or via a rendezvous approach together with ERCP.
Pancreatic duct EUS-guided drainage is technically more challenging than bile duct drainage, with poorer general clinical and technical outcomes (78%) and a higher overall complications rate (7–55%), especially when the transmural route is used due to the need of more aggressive dilation [28, 32].
Anatomical Changes and Interluminal Anastomosis
Another example of true complementarity between the two techniques has emerged for the recently widespread surgical treatment of obesity. In fact, there is an increasing number of patients in whom performing an ERCP is not anatomically possible. This is the case for patients submitted to a Roux-en-Y gastric bypass in whom, due to the exclusion of the distal stomach and proximal duodenum, the ampulla cannot be accessed via the standard ERCP procedure. Single or double balloon enteroscopy is an option for these patients; nevertheless, the success rates are variable. Recently, Kedia et al. [33] described the use of a lumen-apposing metal stent to provide access to the excluded gastrointestinal tract lumen for passage of the duodenoscope for ERCP. This procedure consists in an EUS-guided creation of a gastrogastric or jejunogastric fistula via the placement of a lumen-apposing metal stent. In the series of Kedia et al. [33], the procedure was successful in all 5 cases studied. Larger studies are needed to further assess efficacy, safety, and long term follow-up.
Ampulloma
Adenomatous lesions of the ampulla are entities that also frequently require sequential use of EUS and ERCP. EUS is useful in the differential diagnosis of prominent ampullas, as the majority of these situations represent adenomas or adenocarcinomas. It is also very important in determining the endoscopic resectability of these lesions, having high accuracy in the evaluation of duodenal wall layer invasion as well as biliary and pancreatic intraductal extension [34, 35]. When endoscopic papillectomy is considered, ERCP plays a major role in minimizing the risk of postprocedure pancreatitis by placing a prophylactic pancreatic plastic stent. On the other hand, in advanced and/or unresectable lesions, ERCP is the procedure of choice for palliation of obstructive jaundice [36].
Fluid Collection Drainage
Finally, another situation that can benefit from this convergence of interests between EUS and ERCP is that of pancreatic/peripancreatic fluid collections. Endoscopy, with or without EUS guidance, is nowadays considered the first therapeutic option for transmural drainage of postpancreatitis pseudocysts and for walling off necrosis, being effective and safe, with lower morbidity and mortality when compared to surgical management [37]. Nevertheless, when collections are associated with a disconnected pancreatic duct, EUS-transmural drainage is often incomplete and permanent stenting may be required. In these situations, a combined approach with ERCP can be very useful and highly effective, especially in the presence of a partial pancreatic duct disruption that can be successfully bridged through the papilla [38, 39].
EUS-ERCP: Convergence in a Single Session?
As mentioned before, nowadays more and more clinical situations have an indication to perform both EUS and ERCP. In fact, these two techniques are often required at the same time for the same patient. Combining both in a single session is not yet common in routine care and they are usually performed on separate days. One of the major reasons for this is the lack of an appropriate facility as logistically adapted unit of endoscopy along with the absence of a high enough number of well-trained professionals in both procedures. Nevertheless, evidence exists that this reality is about to change and, by now, more than 1,000 cases of single-session EUS and ERCP have already been reported in the literature. It is important to notice that these procedures should be performed in centers that have a significant volume for proper management.
The majority of studies have shown that this strategy, using the same sedation, is effective, safe, shortens hospital stay, and improves patient comfort, being, undoubtedly, clinical and financially cost-effective [4].
EUS Segmentation
With the technical evolution of EUS observed in the last decade, the demand for this procedure has increased significantly in several fields besides biliopancreatic pathology, in particular in the local staging of malignant and premalignant lesions of the digestive tract and in the evaluation of pelvic structures, mainly in patients with anal disorders. These facts can raise the question of the benefit of segmentation of endoscopists in these three areas.
In the authors opinion, in tertiary referral centers with the possibility of subdifferentiation into different endoscopic techniques, the presence of endoscopists dedicated to performing EUS in a segmented way can be significant, theoretically with greater expertise. In addition, considering the relevance of endoscopy units prepared for joint implementation of EUS and ERCP, this can make the most of the endoscopists entirely dedicated to both techniques. Similarly, endoscopists dedicated to endoscopic resection of malignant and premalignant gastrointestinal lesions by endoscopic mucosal resection or submucosal dissection may benefit from expertise in EUS in performing local staging of these lesions. Finally, in patients with anal pathology, performing transanal EUS can assist in the evaluation of anatomical abnormalities, such as septic processes or sphincter injuries, and can be a routine clinical tool in proctology consultation for physicians dedicated to anorectal disorders.
Nevertheless, in the authors view, there will always be room for endoscopists who are separately dedicated to these techniques. On the one hand, the older endoscopists, dedicated to ERCP, may not have the disposal for the EUS learning requirements, especially with its long learning curve. On the other hand, the presence of endoscopists dedicated only to EUS in its various indications will always be necessary, namely in centers without the logistic conditions for ERCP, either alone or in association with EUS. In addition, not all clinicians feel comfortable performing advanced interventional procedures, especially because of the technical and physical requirements implicated.
Future Challenges
Apart from the expected continued technological development of equipment and accessories needed for the performance of both techniques, the future will imply, above all, a critical evolution in the training and developing skills of new gastroenterologists.
When these techniques were born, we observed the differentiation of its pioneers and their followers in each of them. With time, the ERCP experts looked to EUS as an important input for day-to-day practice and began to learn it. On the other hand, EUS experts, especially those dedicated to biliopancreatic pathology, also realized that gaining experience in ERCP could bring an important advantage in the performance of the more advanced interventional techniques.
Taking this into account and having in mind both the extremely demanding learning curves and safety as an issue of concern, we believe in a future where the convergence concept will also be seen in the training of upcoming endoscopists.
In our opinion, the modern gastrointestinal fellowships should recognize different levels of training and different types of endoscopists. Those particularly interested in this subset of diseases should train specifically both EUS and ERCP. We certainly believe that this is the way that will allow the continuous improvement of diagnostic and therapeutic pancreaticobiliary endoscopy.
References
1 Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, et al: ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005;62:1–8. [ Links ]
2 ASGE Standards of Practice Committee, Early DS, Ben-Menachem T, Decker GA, Evans JA, Fanelli RD, et al: Appropriate use of GI endoscopy. Gastrointest Endosc 2012;75:1127–1131. [ Links ]
3 Dhir V, Paramasivam RK, Lazaro JC, Maydeo A: The role of therapeutic endoscopic ultrasound now and for the future. Expert Rev Gastroenterol Hepatol 2014;8:775–791. [ Links ]
4 Gornals JB, Esteban JM, Guarner-Argente C, Marra-Lopez C, Repiso A, Sendino O, et al: Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography: can they be successfully combined? Gastroenterol Hepatol 2016;39:627–642. [ Links ]
5 Chapman CG, Siddiqui UD: New scopes, new accessories, new stents for interventional endoscopic ultrasound. Clin Endosc 2016;49:41–46. [ Links ]
6 Venkatachalapathy S, Nayar MK: Therapeutic endoscopic ultrasound. Frontline Gastroenterol 2017;8:119–123. [ Links ]
7 Wani S, Hall M, Wang AY, DiMaio CJ, Muthusamy VR, Keswani RN: Variation in learning curves and competence for ERCP among advanced endoscopy trainees by using cumulative sum analysis. Gastrointest Endosc 2016;83:711–719.e11. [ Links ]
8 Adler DG, Lieb JG 2nd, Cohen J, Pike IM, Park WG, Rizk MK, et al: Quality indicators for ERCP. Gastrointest Endosc 2015;81:54– 66. [ Links ]
9 Ekkelenkamp VE, Koch AD, Rauws EA, Borsboom GJ, de Man RA, Kuipers EJ: Competence development in ERCP: the learning curve of novice trainees. Endoscopy 2014;46:949–955. [ Links ]
10 Wani S, Wallace MB, Cohen J, Pike IM, Adler DG, Kochman ML, et al: Quality indicators for EUS. Gastrointest Endosc 2015;81:67–80. [ Links ]
11 Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, et al: Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy 2012;44:190–206. [ Links ]
12 ASGE Standards of Practice Committee, Maple JT, Ikenberry SO, Anderson MA, Appalaneni V, Decker GA, et al: The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc 2011;74:731–744. [ Links ]
13 Verma D, Kapadia A, Eisen GM, Adler DG: EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006;64:248–254. [ Links ]
14 Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P: EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008;67:235–244. [ Links ]
15 Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, et al: Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol 2007;5:616–623. [ Links ]
16 Polkowski M, Regula J, Tilszer A, Butruk E: Endoscopic ultrasound versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a randomized trial comparing two management strategies. Endoscopy 2007;39:296–303. [ Links ]
17 Benjaminov F, Stein A, Lichtman G, Pomeranz I, Konikoff FM: Consecutive versus separate sessions of endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) for symptomatic choledocholithiasis. Surg Endosc 2013;27:2117–2121. [ Links ]
18 Gornals JB, Moreno R, Castellote J, Loras C, Barranco R, Catala I, et al: Single-session endosonography and endoscopic retrograde cholangiopancreatography for biliopancreatic diseases is feasible, effective and cost beneficial. Dig Liver Dis 2013;45:578–583. [ Links ]
19 Iles-Shih L, Hilden K, Adler DG: Combined ERCP and EUS in one session is safe in elderly patients when compared to non-elderly patients: outcomes in 206 combined procedures. Dig Dis Sci 2012;57:1949–1953. [ Links ]
20 Vohra S, Holt EW, Bhat YM, Kane S, Shah JN, Binmoeller KF: Successful single-session endosonography-based endoscopic retrograde cholangiopancreatography without fluoroscopy in pregnant patients with suspected choledocholithiasis: a case series. J Hepatobiliary Pancreat Sci 2014;21:93–97. [ Links ]
21 Rocca R, De Angelis C, Castellino F, Masoero G, Daperno M, Sostegni R, et al: EUS diagnosis and simultaneous endoscopic retrograde cholangiography treatment of common bile duct stones by using an oblique-viewing echoendoscope. Gastrointest Endosc 2006;63:479–484. [ Links ]
22 Lee JH, Salem R, Aslanian H, Chacho M, Topazian M: Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol 2004;99:1069–1073. [ Links ]
23 Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, et al: Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol 2004;2:209–213. [ Links ]
24 Fritscher-Ravens A, Broering DC, Sriram PV, Topalidis T, Jaeckle S, Thonke F, et al: EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc 2000;52:534–540. [ Links ]
25 Byrne MF, Gerke H, Mitchell RM, Stiffler HL, McGrath K, Branch MS, et al: Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy 2004;36:715–719. [ Links ]
26 Yoon WJ, Brugge WR: Endoscopic evaluation of bile duct strictures. Gastrointest Endosc Clin N Am 2013;23:277–293. [ Links ]
27 Weber A, von Weyhern C, Fend F, Schneider J, Neu B, Meining A, et al: Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol 2008;14:1097–1101. [ Links ]
28 Kahaleh M, Artifon ELA, Perez-Miranda M, Gaidhane M, Rondon C, Itoi T, et al: Endoscopic ultrasonography guided drainage: summary of consortium meeting, May 21, 2012, San Diego, California. World J Gastroenterol 2015;21:726–741. [ Links ]
29 Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK: Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43:102–106. [ Links ]
30 Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR: Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898–900. [ Links ]
31 Iwashita T, Doi S, Yasuda I: Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol 2014;7:94–102. [ Links ]
32 Fabbri C, Luigiano C, Lisotti A, Cennamo V, Virgilio C, Caletti G, et al: Endoscopic ultrasound-guided treatments: are we getting evidence based – a systematic review. World J Gastroenterol 2014;20:8424–8448. [ Links ]
33 Kedia P, Tyberg A, Kumta NA, Gaidhane M, Karia K, Sharaiha RZ, et al: EUS-directed transgastric ERCP for Roux-en-Y gastric bypass anatomy: a minimally invasive approach. Gastrointest Endosc 2015;82:560–565. [ Links ]
34 Manta R, Conigliaro R, Castellani D, Messerotti A, Bertani H, Sabatino G, et al: Linear endoscopic ultrasonography vs magnetic resonance imaging in ampullary tumors. World J Gastroenterol 2010;16:5592–5597. [ Links ]
35 Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK: Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound 2009;37:18–25. [ Links ]
36 Dumonceau JM, Rigaux J, Kahaleh M, Gomez CM, Vandermeeren A, Devière J: Prophylax-is of post-ERCP pancreatitis: a practice survey. Gastrointest Endosc 2010;71:934–939, 939.e1–e2. [ Links ]
37 Singhal S, Rotman SR, Gaidhane M, Kahaleh M: Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc 2013;46:506–514. [ Links ]
38 Shrode CW, Macdonough P, Gaidhane M, Northup PG, Sauer B, Ku J, et al: Multimodality endoscopic treatment of pancreatic duct disruption with stenting and pseudocyst drainage: how efficacious is it? Dig Liver Dis 2013;45:129–133. [ Links ]
39 Trevino JM, Tamhane A, Varadarajulu S: Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol 2010;25:526–531. [ Links ]
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Pedro Moutinho-Ribeiro
Gastroenterology Department, Centro Hospitalar São João
Alameda Prof. Hernâni Monteiro
PT–4200-319 Porto (Portugal)
E-Mail pmoutinhoribeiro@gmail.com
Received: May 24, 2017; Accepted after revision: September 14, 2017














