Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.5 Lisboa out. 2018
https://doi.org/10.1159/000485347
ORIGINAL ARTICLE
Evaluation of the Usefulness of Virtual Chromoendoscopy with Different Color Modes in the MiroCam® System for Characterization of Small Bowel Lesions
Avaliação da Utilidade da Cromoendoscopia Virtual no Sistema MiroCam® para Caracterização de Lesões Elementares do Intestino Delgado
Joana Ribeiro da Silva, Rolando Pinho, Adélia Rodrigues, Ana Ponte, Jaime Rodrigues, Mafalda Sousa, João Carvalho
Department of Gastroenterology, Centro Hospitalar Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal
* Corresponding author.
ABSTRACT
Background: Virtual chromoendoscopy (VC) in small bowel capsule endoscopy can improve the visualization and characterization of different small bowel lesions (SBLs). There are few studies of its usefulness in the Given® system, and there is no evidence yet of its utility in the MiroCam® system. Aim: The aim of this study was to evaluate whether VC can improve the characterization of SBLs with the MiroCam® system. Methods: Twenty-two patients were selected, in which 100 elementary lesions were identified, including erosions (n = 45), ulcers (n = 17), and angioectasias (n = 38). For each lesion identified, images were captured without chromoendoscopy (normal image [NI]) and with chromoendoscopy modes 1 (color mode [CM] 1), 2 (CM2), and 3 (CM3). A score from 1 to 4 was assigned to each image, in which a better evaluation of the characteristics and limits of the lesion was classified in ascending order, where 1 is the worst and 4 the best evaluation. The scores of the various modes were compared with Kendalls tau-c coefficient. Results: The average scores attributed to the photographs in NI, CM1, CM2, and CM3 were 3.83, 2.89, 1.85, and 1.43, respectively (tau-c = –0.75, p < 0.001). Evaluating the elementary lesions independently, the average scores for modes NI, CM1, CM2, and CM3 were 3.83, 2.92, 1.86, and 1.38 (tau-c = –0.77, p < 0.001) for erosions, respectively; 3.87, 2.96, 1.76, and 1.40 (tau-c = –0.80, p < 0.001) for ulcers, respectively; and 3.81, 2.82, 1.87, and 1.50 (tau-c = –0.71, p < 0.001) for angioectasias, respectively. Conclusions: VC using the CMs available in the Miro-Cam® system has not proven useful for a better assessment of any of the SBLs.
Keywords: Virtual chromoendoscopy, MiroCam® system, Small bowel capsule endoscopy, Elementary lesions
RESUMO
Introdução: A cromoendoscopia virtual na capsula endoscópica (CE) tem como principal intuito melhorar a visualização e caracterização de diferentes lesões do intestino delgado. Existem poucos estudos da sua utilidade no sistema Given®, não existindo contudo evidência da sua utilidade no sistema MiroCam®. Objetivos: Avaliar a utilidade da cromoendoscopia virtual na caracterização de lesões elementares do intestino delgado no sistema MiroCam®. Métodos: Avaliados 22 doentes, nos quais foram identificadas 100 lesões elementares, nomeadamente erosões (n: 45), úlceras (n: 17) e angiectasias (n: 38). Para cada lesão identificada, foram captadas imagens sem cromoendoscopia (imagem normal), e com cromoendoscopia nos modos 1 (color mode 1), 2 (color mode 2) e 3 (color mode 3). Atribuída a cada imagem uma pontuação de 1 a 4, na qual uma melhor avaliação das características e limites da lesão se encontra classificada de forma crescente, sendo que 1 corresponde à pior e o 4 à melhor avaliação. As pontuações dos vários modos foram comparadas pelo coeficiente de correlação de Kendall tau-c. Resultados: A pontuação média atribuida às fotografias nos modos imagem normal (NI), color mode 1 (CM1), color mode 2 (CM2) e color mode 3 (CM3) foi respectivamente 3.83, 2.89, 1.85 e 1.43 (tau-c = –0.75, p < 0.001). Avaliando as lesões elementares independentemente, os scores médios para os modos NI, CM1,CM2 e CM3 foram: 3.83, 2.92, 1.86, 1.38 (tau-c = –0.77, p < 0.001) para erosões; 3.87, 2.96, 1.76, 1.40 (tau-c = –0.80, p < 0.001) para úlceras; 3.81, 2.82, 1.87, 1.50 (tau-c = –0.71, p < 0.001) para angiectasias. Conclusões: A cromoendoscopia virtual não se revelou útil para uma melhor avaliação de qualquer uma das lesões elementares.
Palavras-Chave: Cromoendoscopia virtual, Sistema MiroCam®, Capsula endoscópica,·Lesões elementares
Introduction
Capsule endoscopy (CE) was introduced in clinical practice in the year 2001 [1] and allows the noninvasive observation of small intestinal mucosa with a higher diagnostic yield as compared to that of other small bowel imaging modalities [2–5]. To improve the diagnosis and characterization of lesions through the gastrointestinal tract, virtual chromoendoscopy (VC) systems were developed, whose main purpose is both to increase the detection and to improve the visualization and characterization of different small bowel lesions (SBLs) [6, 7].
There are several types of VC in conventional endoscopy: narrow band imaging (Olympus, Tokyo, Japan), Fuji Intelligent Color Enhancement (FICE; Fujinon Inc., Saitama, Japan), and I-Scan (Pentax, Tokyo, Japan) [6]. The use of VC in combination with magnification and high-resolution images enables the inspection of microvascular and surface patterns and circumvents some limitations of conventional chromoendoscopy.
The FICE chromoendoscopy software has recently been incorporated into the new RAPID 6.0 video CE workstation (Given® Imaging Ltd., Yoqneam, Israel) [8–10]. FICE technology decomposes images by specific wavelengths (red, green, and blue) and then directly reconstructs the images with enhanced surface contrast. This leads to the enhancement of tissue microvasculature as a result of the differential optical absorption of light by hemoglobin in the mucosa [6]. On the other hand, blue mode imaging uses a shift of the color within a short wavelength range of 490 to 430 nm superimposed on regular white light. Both these technologies provide real-time enhancement of the surface patterns and color gradients of the gastrointestinal mucosa with the intent to better perceive small differences between adjacent mucosal areas [11]. However, the use of FICE in CE, as well as the ideal settings for better recognition of the various lesions that can be found through the small bowel, is still poorly studied.
Concerning the MiroCam® capsule, there are no published studies evaluating the usefulness of VC. There is only 1 study that has been published as an abstract evaluating the Augmented Live-Body Image Color-Spectrum Enhancement (ALICE) chromoendoscopy system, which showed that it improved the visibility of lesions, such as angioectasias, erosions, and ulcers. There is another image chromoendoscopy system in the MiroCam® system that enhances the image in 3 color modes (CM1, CM2, and CM3), but the potential benefits of this technique in the MiroCam® system have not yet been evaluated and remain uncertain [12].
The aim of this study was to evaluate the potential benefits of VC using the different CMs available in the Miro-Cam® system for the delineation and characterization of SBLs when compared to conventional CE images.
Material and Methods
Type of Study and Patient Selection
A retrospective study was carried out including 62 consecutive patients who underwent CE between January and October 2015 at the Department of Gastroenterology, regardless of the indication of the examination.
Procedures
From the total sample, 22 patients were selected in which elementary lesions were identified, consisting of angioectasias, erosions, and ulcers. The clinical files were retrieved and reviewed to collect patient-related and procedure-related data. Patient variables included age, sex, medical history, and medications. CE-related variables included the indication for the procedure and its report, which included all endoscopic findings.
All CEs were performed at the same center using the Miro-Cam® CE system (MiroCam®; IntroMedic, Seoul, Korea). This capsule records images at a fixed rate of 3 frames per second that are transmitted to an external recorder through human body communication technology. Patients underwent a CE protocol [13] that consists of ingestion of the capsule with a glass of water at 8 a.m. after ingestion of a liquid diet in the last 24 h and an overnight fast; evaluation of the location of the CE using real-time views at 1 and 2 h after ingestion; administration of 10 mg metoclopramide if the capsule remained in the stomach after 2 h; ingestion of a light diet 4 h after the beginning of the procedure and resumption of normal daily activities on an outpatient basis during the day; removal of the recorder 12 h after capsule ingestion (or earlier if real-time viewing confirms that the capsule has already reached the colon).
All CEs, corresponding to the 22 patients, have been visualized under conventional white light by gastroenterologists with experience in small bowel CE (SBCE). From these 22 CEs, 100 lesions were selected for the study, including vascular lesions – angioectasias (n = 38) and inflammatory lesions – erosions (n = 45), and ulcers (n = 17).
Then, 1 of the authors reviewed all CE videos of the 22 patients, in which all CE findings were already marked from the previous white light examination. The images containing the lesions were extracted, de-identified, sequentially numbered, and inserted in a PowerPoint presentation. For each selected lesion, images were taken without chromoendoscopy (normal image [NI]) and with chromoendoscopy in modes 1 (CM1), 2 (CM2), and 3 (CM3).
One hundred images were projected on a screen in a darkened room for the CE readers involved in this study: 3 gastroenterology fellows and 3 gastroenterology assistants, all with experience in SBCE. In each projection, 4 images of the same lesion were presented: NI, CM1, CM2, and CM3 (Fig. 1, 2, 3). The evaluation of the images was therefore, performed simultaneously and under the same conditions by each CE reader.
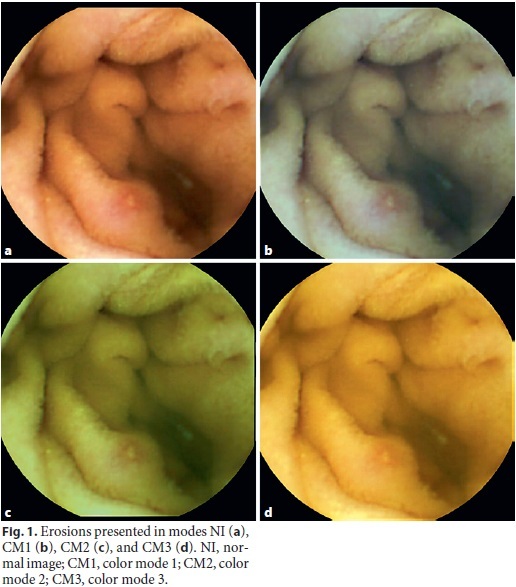
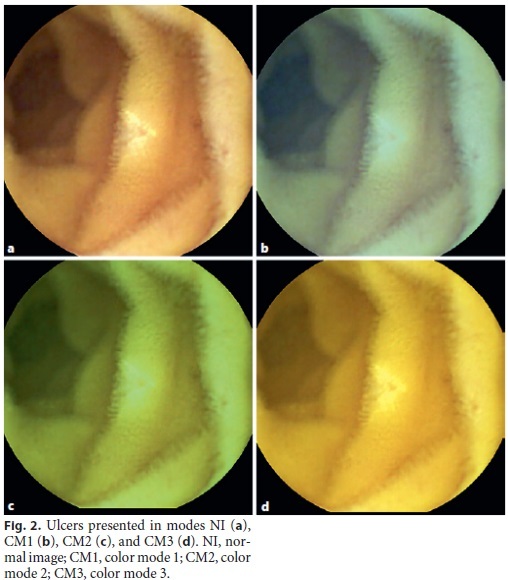
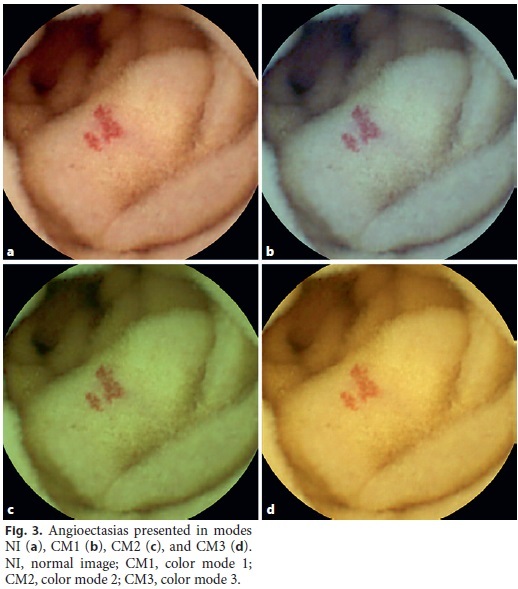
Each reader attributed a score of 1–4 to each image, in which a better evaluation of the characteristics and limits of the lesion was classified increasingly, with 1 being the worst and 4 being the best evaluation. All these data were recorded on a sheet previously supplied to the readers. This sheet had a table in which each line referred to the 100 identified lesions and the 4 columns referred to modes NI, CM1, CM2, and CM3. After the projection and grading of all images was finished, an author collected all sheets for subsequente statistical analysis.
Statistical Analysis
Descriptive results are presented as medians and interquartile ranges for continuous variables and proportions for categorical variables. The scores of the various CMs were compared using Kendalls tau-c correlation coefficient.
Results were considered significant at p < 0.05. The Statistical Package for the Social Sciences, version 20.0 (IBM Corp., Armonk, NY, USA), was used for data entry and data analysis.
Results
Visualization of the entire small bowel was achieved in all 22 CE examinations. Regarding bowel preparation, it was considered poor in 9 patients, excellent in 6, fair in 4, and good in 3 using the Qualitative Classification of Brotz [14, 15].
There was a total of 22 patients, 59.1% (n = 13) were males, with a median age of 58 years. The most frequent indication for CE was obscure gastrointestinal bleeding (OGIB). Patient demographics and clinical characteristics, as well as CE indications and respective elementary lesions, are summarized in Table 1.
The average scores attributed to the CE images in NI, CM1, CM2, and CM3 were, respectively, 3.83, 2.89, 1.85, and 1.43 (tau-c = –0.75, p < 0.001; Table 2). Evaluating the elementary lesions independently, the average scores for modes NI, CM1, CM2, and CM3 were 3.83, 2.92, 1.86, and 1.38 (tau-c = –0.77, p < 0.001) for erosions, respectively; 3.87, 2.96, 1.76, and 1.40 (tau-c = –0.80, p < 0.001) for ulcers, respectively; and 3.81, 2.82, 1.87, and 1.50 (tau-c = –0.71, p < 0.001) for angioectasias, respectively (Table 2).
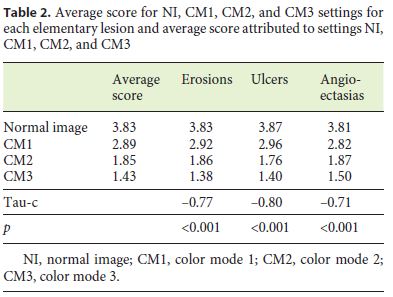
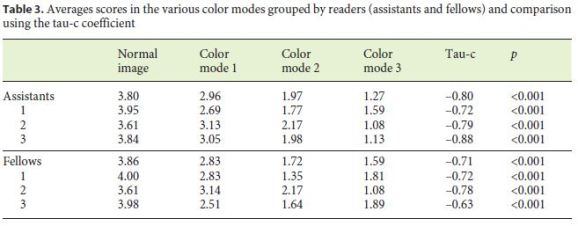
Table 3 shows the individual values for each of the CMs of each reader, grouped as gastroenterology fellows and assistants, as well as the averages per group of fellows and assistants. The comparison of the scores in each visualization mode is also presented using Kendalls tau-c coefficient.
Discussion
CE is an excellent small bowel examination technique that in the proper clinical context provides a diagnostic yield ranging from 55 to 81% [16]. Current CE devices are passively propelled through gut peristalsis, which conveys some limitations to this examination. Consequently, visualization of the entire length of the small bowel is achieved in 80–90% of patients [17]. On the other hand, uncontrollability of the movement of the capsule leads to up to 30% of discrete lesions being missed, especially when there is increased gut motility and/or when the image of a lesion is captured in only 1 frame [17]. Moreover, some lesions may not be photographed at all, as some areas of the small bowel mucosa may not be captured by the CE system. Furthermore, CE has other technical limitations, including the lack of control and the impossibility of detailed examination of the lesion in selected angles [18], the lack of therapeutic capabilities, and the inability to obtain biopsies [1].
Hence, it is critical to obtain the maximum of information possible from the images that are available. In this setting, technologies like chromoendoscopy could be of paramount importance. The goal will be in the near future to overcome these limitations and develop a new generation of CE able to perform both diagnostic and therapeutic procedures, a role currently reserved for device-assisted enteroscopy [19].
The main clinical indications for CE are investigation of OGIB, assessment of known or suspected small bowel Crohn disease, small bowel tumors, hereditary polyposis syndrome, and coeliac disease [20]. The role of CE in the study of OGIB is supported by its higher diagnostic yield when compared to other imaging modalities [21, 22] and by the safety and ease with which it is performed.
To improve the identification, diagnosis, and characterization of gastrointestinal lesions, several VC systems were developed. Globally, the purpose is to enhance tissue microvasculature as a result of the differential optical absorption of light by hemoglobin in the mucosa. There are several types of VC, e.g., FICE, the software recently incorporated in the Given® system, one of the most studied. However, clinical judgment and scientific evidence remain divided as to its clinical usefulness [17].
Imagawa et al. [9] conducted a study to clarify whether visualization of SBLs is improved by FICE image analysis. The authors evaluated 143 lesions, i.e., angioectasias (n = 23), erosions/ulcerations (n = 45), and tumors (n = 75), and concluded that FICE improves the visibility of various SBLs, with principal relevance for the detection of angioectasias.
Nakamura et al. [23] evaluated the usefulness of FICE in detecting angioectasias. They concluded that the sensitivity and specificity of conventional reading for detecting angioectasias were 80 and 100%, respectively. When the reading was done with FICE, the sensitivity increased to 91%, while specificity was reduced to 86%.
Sakai et al. [24] also showed that FICE 1 and 2 contribute to a significant improvement in the detection of angioectasias, erosions, and ulcers.
Cotter et al. [25] evaluated whether VC can improve the delineation of SBLs previously detected by conventional white-light SBCE. In their study, 100 lesions were reviewed using the 3 FICE settings and the blue filter. Overall, the delineation of lesions was improved in 77% of cases with FICE 1, in 74% with FICE 2, in 41% with FICE 3, and in 39% with the blue filter.
Sato et al. [26] showed that FICE adds valuable information to the conventional image and adds advantages inthe diagnosis of SBL, such as angioectasias, erosions, and ulcers, especially with the use of FICE in configurations 1 and 2.
Dias de Castro et al. [27] examined how FICE can improve the detection of potentially bleeding lesions in patients presenting with OGIB whose SBCE examinations were negative. A total of 42 patients were included in this study; 16 patients experienced rebleeding after a mean follow-up of 26 months. Review of SBCE images using FICE 1 enabled the identification of previously unrecognized P2 lesions (lesions with high bleeding potential), mainly angioectasias in 9 patients, and P1 lesions (lesions with uncertain bleeding potential), mainly erosions in 26 patients (62%). They concluded that the visualization of CE with FICE 1 may be a valuable tool in patients with OGIB and a negative SBCE, particularly for those who experience rebleeding.
However, not all studies are in agreement with the benefits of the FICE. Gupta et al. [28] investigated the potential benefit of FICE-assisted SBCE for the detection and characterization of SBLs in patients with OGIB. This study concluded that FICE was not better than white light for diagnosing and characterizing lesions on SBCE in the setting of OGIB.
Yung et al. [17] performed a meta-analysis whose objective was to evaluate if FICE contributes to the improvement of lesion detection comparing all FICE settings. Thirteen studies were analyzed, and, overall, the use of the 3 FICE modes did not significantly improve the detection rate or the quality of visualization of the most common pathological findings seen on SBCE. Therefore, the results reported in the literature are limited, discordant, and often contradictory regarding the accuracy and clinical value of VC, and the optimal settings to improve the detection and delimitation of different types of lesions are not yet known.
Although there are several studies that evaluate the use of VC in the Given® CE system, there is only 1 published study, in abstract form, to evaluate chromoendoscopy in the MiroCam® system, in this case analyzing ALICE chromoendoscopy. Ryu et al. [12] examined whether the visibility with the various settings available using ALICE was dependent on the type of SBL. The lesions that were evaluated were classified as elevated (tumor and polyp), flat (angioectasias and erosion), and depressed (ulcer). They concluded that ALICE improves visibility of flat and depressed lesions in the small bowel.
Our study is the first that evaluates the potential advantages of VC with the various color modes as an auxiliary method in the delineation and characterization of SBLs in the MiroCam® system. In our study, VC was not useful for a better evaluation and characterization of any of the elementary lesions. The findings were consistent among all readers, both in the assistants and fellows groups, suggesting that the findings were not dependent on the experience of the readers. Among all VC CMs, CM1 proved to be better than CM2, and the latter better than CM3.
This study has some limitations. First of all, this study was not aimed at evaluating the diagnostic yield of the different CMs relative to the NI. Accordingly, although the different CMs could not help in the delineation and characterization of the different elementary findings, this study was not designed to demonstrate an eventual diagnostic gain over the NI. In addition, the NI was used to select the elementary lesions to be evaluated in the 3 CMs. Another potential drawback is the fact that static images were evaluated instead of a standard CE video. The lesions were evaluated simultaneously with or without chromoendoscopy. Although this is useful to compare the different CMs, it may also add some bias to the comparison. Although the results were already statistically significant, a larger sample of images could be useful. Finally, the images were graded from 1 to 4 depending on the quality of the characterization of the lesion in the image. Another grading system could be used to independently compare each CM to the NI as a better, worse, or similar characterization of the lesion. Although this subjective scale could be better to independently compare the different CMs to the NI, it could not rank the different CMs relative to each other.
More evidence is necessary to clarify the real value of VC systems in CE, particularly that of the MiroCam® system, and to determine the best indications for this modality in order to define the role of this technology in CE.
References
1 Singeap AM, Stanciu C, Trifan A: Capsule endoscopy: the road ahead. World J Gastroenterol 2016; 22: 369–378. [ Links ]
2 Dionisio PM, Gurudu SR, Leighton JA, et al: Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohns disease: a meta-analysis. Am J Gastroenterol 2010;105: 1240–1248. [ Links ]
3 Chen X, Ran ZH, Tong JL: A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel disease. World J Gastroenterol 2007; 13: 4372–4378. [ Links ]
4 Apostolopoulos P, Liatsos C, Gralnek IM, et al: The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy 2006; 38: 1127–1132. [ Links ]
5 Lewis BS, Swain P: Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding. Results of a pilot study. Gastrointest Endosc 2002; 56: 349–353. [ Links ]
6 Spada C, Hassan C, Costamagna G: Virtual chromoendoscopy: will it play a role in capsule endoscopy? Dig Liver Dis 2011; 43: 927–928. [ Links ]
7 Gossum AV: Image-enhanced capsule endoscopy for characterization of small bowel lesions. Best Pract Res Clin Gastroenterol 2015;29: 525–531. [ Links ]
8 Imagawa H, Oka S, Tanaka S, et al: Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol 2011; 46: 1133–1137. [ Links ]
9 Imagawa H, Oka S, Tanaka S, et al: Improved visibility of lesions of the small intestine via capsule endoscopy with computed virtual chromoendoscopy. Gastrointest Endosc 2011; 73: 299–306. [ Links ]
10 Duque G, Almeida N, Figueiredo P, et al: Virtual chromoendoscopy can be a useful software tool in capsule endoscopy. Rev Esp Enferm Dig 2012; 104: 231–236. [ Links ]
11 Rimbas M, Zahiu DCM, Voiosu AM, et al: Is virtual chromoendoscopy useful in the evaluation of subtle ulcerative small-bowel lesions detected by video capsule endoscopy? Endosc Int Open 2015; 3:E615–E620. [ Links ]
12 Ryu C, Song J, Lee M, et al: Does capsule endoscopy with ALICE improve visibility of small bowel lesions? Gastrointest Endosc 2013; 77:AB466. [ Links ]
13 Ponte A, Pinho R, Rodrigues A, et al: Validation of the computed assessment of cleansing score with the MiroCam® system. Rev Esp Enferm Dig 2016; 108: 709–715. [ Links ]
14 Brotz C, Nandi N, Conn M, et al: A validation study of 3 grading systems to evaluate smallbowel cleansing for wireless capsule endoscopy: a quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest Endosc 2009; 69: 262–270. [ Links ]
15 Ponte A, Pinho R, Carvalho J, et al: Review of small-bowel cleansing scales in capsule endoscopy: a panoply of choices. World J Gastrointest Endosc 2016; 8: 600–609. [ Links ]
16 Ahmad NA, Iqbal N, Joyce A: Clinical impact of capsule endoscopy on management of gastrointestinal disorders. Clin Gastroenterol Hepatol 2008; 6: 433–437. [ Links ]
17 Yung DE, Carvalho P, Giannakou A, et al: Clinical validity of flexible spectral imaging color enhancement (FICE) in small-bowel capsule endoscopy: a systematic review and meta-analysis. Endoscopy 2017; 49: 258–269. [ Links ]
18 Rodrigues JP, Pinho R, Rodrigues A, et al: Validation of SPICE, a method to differentiate small bowel submucosal lesions from innocent bulges on capsule endoscopy. Rev Esp Enferm Dig 2017; 109: 106–113. [ Links ]
19 Pinho R, Mascarenhas-Saraiva M, Mao-de-Ferro S, et al: Multicenter survey on the use of device-assisted enteroscopy in Portugal. United European Gastroenterol J 2016; 4: 264–274. [ Links ]
20 Kopvlov U, Seidman EG: Clinical applications of small bowel capsule endoscopy. Clin Exp Gastroenterol 2013; 6: 129–137. [ Links ]
21 Triester SL, Leighton JA, Leontiadis GI, et al: A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005; 100: 2407–2418. [ Links ]
22 Ribeiro I, Pinho R, Rodrigues A, et al: Obscure gastrointestinal bleeding: which factors are associated with positive capsule endoscopy findings? Rev Esp Enferm Dig 2015; 107: 334–339. [ Links ]
23 Nakamura M, Ohmiya N, Miyahara R, et al: Usefulness of flexible spectral imaging color enhancement (FICE) for the detection of angiodysplasia in the preview of capsule endoscopy. Hepatogastroenterology 2012; 59: 1474–1477. [ Links ]
24 Sakai E, Endo H, Kato S, et al: Capsule endoscopy with flexible spectral imaging color enhancement reduces the bile pigment effect and improves the detectability of small bowel lesions. BMC Gastroenterol 2012; 12: 83. [ Links ]
25 Cotter J, Magalhães J, de Castro FD, et al: Virtual chromoendoscopy in small bowel capsule endoscopy: new light or a cast of shadow? World J Gastrointest Endosc 2014; 6: 359–365. [ Links ]
26 Sato Y, Sagawa T, HiraKawa M, et al: Clinical utility of capsule endoscopy with flexible spectral imaging color enhancement for diagnosis of small bowel lesions Endosc Int Open 2014; 2:E80–E87. [ Links ]
27 Dias de Castro F, Magalhaes J, Boal Carvalho P, et al: Improving diagnostic yield in obscure gastrointestinal bleeding – how virtual chromoendoscopy may be the answer. Eur J Gastroenterol Hepatol 2015; 27: 735–740. [ Links ]
28 Gupta T, Ibrahim M, Deviere J, et al: Evaluation of Fujinon intelligent chromo endoscopy- assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol 2011; 17: 4590–4595. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors declare no conflicts of interest with respect to this article.
* Corresponding author.
Dr. Joana Ribeiro da Silva
Department of Gastroenterology, Centro Hospitalar Vila Nova de Gaia/Espinho
Rua Conceição Fernandes
PT–4434-502 Vila Nova de Gaia (Portugal)
E-Mail joanasilva67@hotmail.com
Received: August 26, 2017; Accepted after revision: October 13, 2017
Funding Sources
The authors declare that they have not received any financial support for this article.
Author Contributions
J.R.S.: Design of the study, analysis and interpretation of the data and references, and drafting of the article. R.P.: Design of the study, analysis and interpretation of the data and references, drafting of the article, and critical revision of the article for important intellectual content. A.R.: Analysis and interpretation of the data and references. A.P.: Analysis and interpretation of the data and references. J.R.: Analysis and interpretation of the data and references. M.S.: Analysis and interpretation of the data and references. J.C.: Final approval of the article.














