Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.5 Lisboa out. 2018
https://doi.org/10.1159/000485429
CLINICAL CASE STUDY
Endoscopic Ultrasound-Guided Biliary Drainage in Two Patients with Difficult Biliary Access
Drenagem biliar guiada por ecoendoscopia em dois casos de acesso biliar difícil
Diogo Libânioa,b, Sílvia Giestasb, David Martinez-Aresb, Frederico Ferreirab, Jorge Canenac, Manuela Certod, Luís Lopesb,e
aGastroenterology Department, Portuguese Oncology Institute of Porto, Porto, bGastroenterology Department, Unidade Local de Saúde Alto Minho, Viana do Castelo, cNova Medical School/Faculdade de Ciências Médicas, University Centre of Gastroenterology, CUF Infante Santo Hospital, Lisboa, dRadiology Department, Centro Hospitalar do Porto, Porto, and eSchool of Medicine, University of Minho, Braga, Portugal
* Corresponding author.
ABSTRACT
Introduction: Endoscopic retrograde cholangiopancreatography is the method of choice for biliary drainage, although in some cases standard biliary access is difficult or even impossible. Endoscopic ultrasound (EUS)-guided endoluminal procedures are an alternative in these cases, although experience with these techniques is still limited. Clinical Case: We present two cases of successful EUS-guided biliary drainage. In the first case, a hepaticogastrostomy was performed in a patient with stage IV gastric adenocarcinoma with obstructive jaundice due to compression of the hilum, where malignant gastric stenosis and previous palliative gastrojejunostomy precluded access to the second part of the duodenum. In the second case, a patient with a pancreatic head adenocarcinoma with duodenal invasion that precluded major papillae identification was submitted to a choledochoduodenostomy. Both procedures occurred without immediate or delayed adverse events, with technical and clinical success. Discussion: Although experience with EUS-guided biliary drainage is still limited, its efficacy and safety is favourable when compared with percutaneous and surgical drainage, and should be considered an alternative to these techniques where sufficient expertise exists.
Keywords: Endoscopic retrograde cholangiopancreatography, Biliary drainage, Endoscopic ultrasonography, Palliative therapy, Gastrointestinal cancer
RESUMO
Introdução: A colangiopancreatografia retrógrada endoscópica é o procedimento de escolha para a drenagem biliar, embora em alguns casos o acesso biliar convencional é difícil ou até impossível. As técnicas de drenagem guiadas por ecoendoscopia são uma alternativa nestes casos, embora a experiência seja ainda limitada. Caso: Apresentamos dois casos de drenagem biliar eficaz guiada por ecoendoscopia. No primeiro caso foi realizada hepaticogastrostomia numa doente com adenocarcinoma gástrico estadio IV, com icterícia obstrutiva devido a compressão hilar pela neoplasia, na qual o acesso à segunda porção duodenal se revelou impossível devido à neoplasia gástrica estenosante e a antecedentes de gastrojejunostomia paliativa. No segundo caso, uma doente com adenocarcinoma cefalo-pancreático com invasão duodenal que impedia a identificação da papila foi submetida a coledocoduodenostomia. Em ambos os procedimentos foi conseguida drenagem biliar eficaz e não ocorreram eventos adversos imediatos ou tardios. Discussão: Apesar de a experiência com técnicas de drenagem biliar guiadas por ecoendoscopia ser limitada, o seu perfil de eficácia e segurança parece ser favorável quando comparada com as alternativas (drenagem percutânea ou cirúrgica), pelo que devem ser consideradas quando exista equipamento e experiência necessária.
Palavras-Chave: Colangiopancreatografia retrógrada endoscópica,·drenagem biliar, ecoendoscopia, terapêutica paliativa, neoplasias gastrointestinais
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is the method of choice for biliary endotherapy, including drainage in obstructive jaundice. However, there are some patients in whom biliary access is difficult or impossible even in expert hands, due to luminal obstruction or surgically modified anatomy (e.g., Rouxen- Y gastrojejunostomy). When faced with difficulties in achieving deep biliary cannulation, clinicians need alternative techniques to allow minimally invasive biliary therapy. The approaches available to overcome these ERCP limitations include endoscopic ultrasound (EUS)-guided endoluminal procedures, balloon-assisted ERCP, percutaneous techniques or surgery, each one with advantages, drawbacks, and different availability. The percutaneous approach is generally more available, but is also associated with a significant risk of adverse events, and is sometimes associated with permanent or temporary external biliary drainage that can impair patients quality of life [1]. On the other hand, EUS-guided procedures have the advantage of being entirely performed intraluminally. EUS-guided therapy includes direct transgastric or transduodenal stenting, rendezvous techniques, and anterograde transpapillary stent insertion, although experience with these techniques is still limited [2]. We report and describe two cases of successful biliary decompression through direct transluminal stenting using EUS guidance.
Clinical Cases
Case 1 – EUS-Guided Hepaticogastrostomy
We report the case of a 60-year-old female patient with stage IV adenocarcinoma of the gastric antrum previously submitted to palliative gastrojejunostomy, and obstructive jaundice due to malignant compression of the hepatic hilum by the gastric neoplasm. The patient presented with obstructive jaundice (total bilirubin 13.2 mg/dL) and CT scan showed severe dilation of the intrahepatic biliary tree caused by obstruction at the hepatic hilum. A gastric selfexpandable noncovered metal stent (Wallflex 22/90 mm; Boston Scientific, Marlborough, MA, USA) was initially placed to allow the passage of the duodenoscope through the stenosis. The distal extremityof the stent was placed in the duodenal bulb with the aim of not impairing access to the papilla, but the second portion of the duodenum could not be reached due to anatomical deformation caused by the tumor and the surgical history. After multidisciplinary discussion and patient information about the palliative therapeutic alternatives, EUS-guided drainage was decided. EUS was then performed with a linear therapeutic echoendoscope (Pentax, EG-3870UTK) and intrahepatic biliary duct dilation was confirmed. With the echoendoscope positioned in the upper part of the lesser curvature, a peripheral intrahepatic bile duct was punctured with a 19-G needle (Expect, Boston Scientific) (Fig. 1a). The success of the biliary puncture was confirmed after observing bile in the needle aspirate, followed by contrast injection into the biliary tree. A 450-cm-long, 0.035-inch guidewire (Jagwire Stiff STTM, Boston Scientific) was then advanced through the needle to the intrahepatic ducts, followed by dilation of the tract with a 6-Fr cystotome (Cysto Gastro Set; Endoflex, GmbH, Voerde, Germany; endocut Q; 40 W; effect 1). A self-expandable fully covered metal stent (Evolution Biliary 10/60 mm, Cook Medical, Bloomington, IN, USA) was then placed from the left intrahepatic duct to the stomach without adverse events (Fig. 1b). Finally, a plastic double pigtail biliary stent (Zimmon 10 Fr × 10 cm, Cook Medical) was placed inside the metal stent to reduce the risk of stent migration (Fig. 1, d). One day after the procedure, the patient developed fever and antibiotherapy (piperacillin/tazobactam) was prescribed for 5 days, after hemocultures were obtained (that revealed to be negative). The fever ceased after 2 days of antibiotherapy, and the patient was discharged 5 days after the procedure without jaundice and without other symptoms. The patient died 2 months after the procedure without related adverse events or jaundice recurrence.
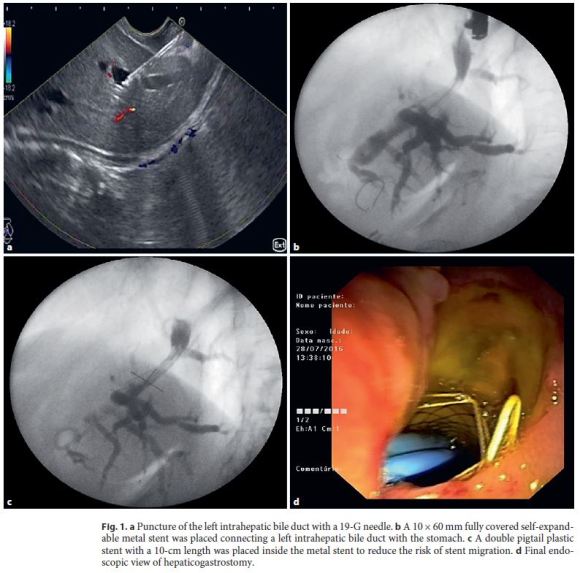
Case 2 – EUS-Guided Choledochoduodenostomy
A 66-year-old female was admitted due to obstructive jaundice (total bilirubin 15.4 mg/dL, direct 8.2 mg/dL) and weight loss, and a pancreatic head cancer was diagnosed. CT scan and EUS showed dilation of the common and intrahepatic bile ducts, a pancreatic mass with vascular invasion (mesenteric and portal veins) and regional adenopathies. EUS-FNA revealed a pancreatic adenocarcinoma. The disease was considered nonresectable and the patient was proposed for palliative chemotherapy, although biliary drainage was necessary before treatment initiation. An ERCP was first attempted but standard access to the biliary tree was not possible due to nonidentification of the papilla associated with duodenal invasion. EUS-guided biliary drainage was then decided after multidisciplinary team evaluation. The dilated common bile duct was punctured by a transbulbar approach with a 19-G needle (Expect, Boston Scientific) and the cholangiogram performed revealed a tight stenosis in the distal portion of the biliary tree (Fig. 2a). A 450-cm-long, 0.035-inch guidewire (JagwireTM, Boston Scientific) was advanced through the needle (Fig. 2b) and the tract was dilated with a 6-Fr cystotome (Cysto Gastro Set; Endoflex, GmbH; endocut 40 W/effect 1). A diablo-shaped self-expandable fully covered metal stent with 20 mm (usable length) × 14/26 mm (lumen/flanges) (Diagmed Healthcare; Thirsk, UK) was finally inserted and deployed, connecting the common bile duct to the duodenal bulb, with successful biliary drainage and without immediate adverse events (Fig. 2c, d). This was followed by resolution of jaundice, and the patient was able to initiate palliative chemotherapy. No late adverse events occurred during the 4 months follow-up and the patient is still alive on chemotherapy.
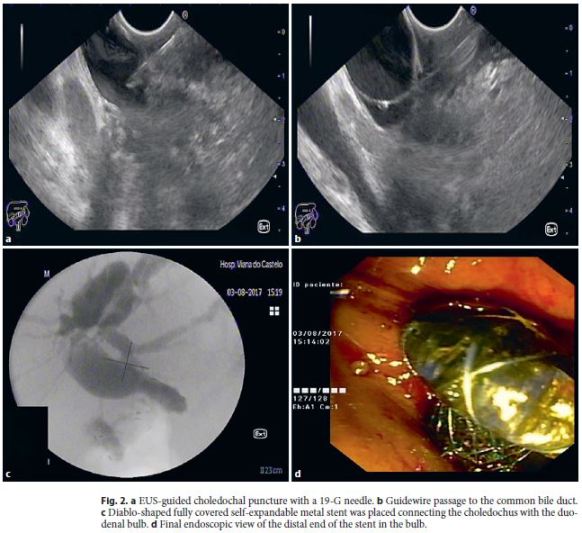
Discussion
In recent years, EUS-guided biliary drainage has emerged as an alternative to percutaneous and surgical drainage in cases where ERCP techniques are difficult or not possible [2]. Here we present two cases of successful EUS-guided biliary drainage through hepaticogastrostomy and choledochoduodenostomy. In the first case, ERCP was not possible due to gastric outlet obstruction and surgical modified anatomy and in the second, pancreatic head cancer invasion into the duodenum wall precluded papilla identification. In both cases, biliary drainage was attained with the insertion of self-expandable metal stents connecting the biliary tree with the stomach in the first case, and the duodenal bulb in the second. In the hepaticogastrostomy case, a plastic biliary stent was also placed inside the metal stent to decrease the risk of stent migration. The stents used were chosen based on anatomical considerations, operators experience as well as availability, since there are no comparative studies evaluating the outcomes of different stent types, due to the novelty of these techniques and the limited number of cases reported. In the first case, a standard fully covered biliary self-expandable metal stent was chosen because diablo-shaped stents are not adequate for left hepaticogastrostomy, as a result of their shorter length and large luminal diameter. We used a stent-in-stent technique (with a longer plastic stent inside the fully covered selfexpandable metal stent) to decrease the risk of stent migration. An alternative to this approach would be the placement of a dedicated half-covered and half-uncovered stent designed specifically for hepaticogastrostomy that was recently evaluated in a retrospective study including 41 patients, although migration still occurred in 4.9% [3]. Diablo-shaped stents, although not appropriate for hepaticogastrostomy, can be used in choledochoduodenostomies where its shape is adequate for appropriate fixation of the duodenal and common bile duct walls. Recently developed lumen-apposing stents designed specifically for biliary drainage are also an option, making this procedure apparently simpler, since tract dilatation is not needed with these devices.
As previously stated, EUS-guided biliary drainage is emerging as an alternative to conventional percutaneous and surgical drainage, although experience with these techniques and their availability is still limited, and comparative studies with conventional techniques are scarce. Even though, EUS-guided drainage seems promising since according to a recent meta-analysis successful biliary drainage is achieved in 93% of the cases, although the rate of adverse events is not negligible (17% overall) [1]. Bile leak is the most frequent adverse event, although other early and late complications can occur. Early complications include cholangitis, bleeding, stent misplacement, and bile leaks [4]. Dilation of the tract before stent insertion is recommended to decrease the risk of bile leaks since it avoids frequent device exchange and decreases procedural time. A cystotome seems to be the instrument of choice to perform the dilation since it was associated with a higher rate of technical success [5], although biliary dilation catheters can also be used. Late complications include stent malfunction due to occlusion as well as stent migration, which has a poor prognosis. For hepaticogastrostomy, long stents may decrease the risk of stent migration. Indeed, a stent length ≥3 cm in the intraluminal portion was shown to be associated with a lower risk of stent migration in a retrospective study including 51 patients [6] and a small study including 4 patients reported no migration when ≥10 cm stents (total length) were used [4]. Choosing a stent with a large diameter is also important to decrease the risk of stent dysfunction due to granulation tissue in the hepatic side of the stent, although the ideal diameter is not well established. Diablo-shaped stents also seem to decrease this risk of stent migration in choledochoduodenostomy and should be used in these cases.
Regarding the comparison between EUS-guided drainage and percutaneous drainage, EUS techniques seem to have a better efficacy and safety profile. Indeed, according to a recent systematic review and meta-analysis, it was associated with a significantly lower rate of drainage failure (OR 0.45, 95% CI 0.23–0.89), post-procedural adverse events (OR 0.23, 95% CI 0.12–0.47) and need for reintervention (OR 0.13, 95% CI 0.07–0.24) [1]. Bile leak was the most frequent adverse event, occurring in 10% of the patients undergoing transcutaneous drainage (vs. 3% in EUS-guided drainage). Rates of procedurerelated bleeding, cholangitis, sepsis, and peritonitis were also slightly higher with the percutaneous approach, while procedure-related death was similar between groups. EUS-guided drainage has also the probable advantage of being associated with a better quality of life since the drainage is always performed without external drains. Although surgical drainage is also an option when minimally invasive procedures fail, to our knowledge there are no studies directly comparing the outcomes of EUS-guided and surgical biliary drainage. In a recent study, EUS-guided biliary drainage was also comparable with conventional transpapillary ERCP drainage in terms of clinical success, procedural time, and adverse events, being even associated with a lower rate of pancreatitis [7]. However, this was a small single-center study and standard ERCP has a demonstrated high efficacy and safety profile with experienced operators and should be the first-line approach to biliary drainage. Regarding patients with surgically altered anatomy, enteroscopy-assisted ERCP is also an alternative, although a recent multicentric comparative study showed that EUS-guided biliary drainage is more effective and safe than enteroscopy-assisted ERCP (technical success 98 vs. 65.3%, p = 0.0001; clinical success 88 vs. 59.1%, p = 0.03; adverse events 4 vs. 20%, p = 0.01) [8].
Concerning the approach of biliary drainage, both the hepatogastric and choledochoduodenal approach seem to have comparable success in attaining biliary drainage, and there are still some controversies (in cases where both approaches are possible). Hepatogastric drainage was associated with a higher risk of complications in a recent review [9] and in a recent multicenter study, where choledochoduodenostomy was also associated with shorter inpatient stay and improved stent patency [10]. On the other hand, a comparative study between the two techniques showed a significantly longer stent patency with hepaticogastrostomy and a lower risk of adverse events [11]. Thus, as no definite conclusions exist concerning the best approach, for now the decision should be made on a case-by-case basis, taking also into account that in certain circumstances the decision is linked to patientrelated factors (e.g., hepaticogastrostomy is only possible if there is a significant dilation of the intrahepatic bile ducts, and choledochoduodenostomy may not be feasible in cases of duodenal invasion, gastric outlet obstruction, or surgically altered anatomy).
In conclusion, we report two cases of successful EUS-guided biliary drainage in cases where ERCP was not possible, raising the importance of training and availability of these techniques in order to provide the patients with the best treatment, although we recognize that these techniques should only be performed wherever adequate expertise exists.
References
1 Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, et al: Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and metaanalysis. Gastrointest Endosc 2017;85:904–914. [ Links ]
2 Liao WC, Angsuwatcharakon P, Isayama H, Dhir V, Devereaux B, Khor CJ, et al: International consensus recommendations for difficult biliary access. Gastrointest Endosc 2017;85:295–304. [ Links ]
3 De Cassan C, Bories E, Pesenti C, Caillol F, Godat S, Ratone JP, et al: Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: results of a retrospective monocentric study. Endosc Ultrasound 2017;6:329–335. [ Links ]
4 Mandai K, Uno K, Okada Y, Suzuki A, Yasuda K: Endoscopic ultrasound-guided hepaticogastrostomy using a 6-F cystotome and 12-cm covered metal stent. Endosc Int Open 2016;4:E287–E291. [ Links ]
5 Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, et al: Multicenter retrospective study of endoscopic ultrasoundguided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci 2014;21:328–334. [ Links ]
6 Ogura T, Yamamoto K, Sano T, Onda S, Imoto A, Masuda D, et al: Stent length is impact factor associated with stent patency in endoscopic ultrasound-guided hepaticogastrostomy. J Gastroenterol Hepatol 2015;30:1748–1752. [ Links ]
7 Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, et al: Endoscopic ultrasound-guided choledochoduodenostomy versus transpapillary stenting for distal biliary obstruction. Endoscopy 2016;48:164–169. [ Links ]
8 Khashab MA, El Zein MH, Sharzehi K, Marson FP, Haluszka O, Small AJ, et al: EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open 2016;4:E1322–E1327. [ Links ]
9 Dhir V, Isayama H, Itoi T, Almadi M, Siripun A, Teoh AYB, et al: Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig Endosc 2017;29:472–485. [ Links ]
10 Khashab MA, Messallam AA, Penas I, Nakai Y, Modayil RJ, De la Serna C, et al: International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. Choledochoduodenostomy approaches. Endosc Int Open 2016;4:E175–E181. [ Links ]
11 Ogura T, Chiba Y, Masuda D, Kitano M, Sano T, Saori O, et al: Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016;48:163. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest do declare.
* Corresponding author.
Dr. Diogo Libânio
Gastroenterology Department, Portuguese Oncology Institute of Porto
Rua Dr. António Bernardino de Almeida
PT–4200-072 Porto (Portugal)
E-Mail diogolibaniomonteiro@gmail.com
Received: October 11, 2017; Accepted after revision: November 16, 2017














