Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.1 Lisboa fev. 2019
https://doi.org/10.1159/000487157
CLINICAL CASE STUDY
Pain and Swelling after Percutaneous Endoscopic Gastrostomy Removal: An Unexpected Evolution
Dor e edema após remoção de gastrostomia percutânea endoscópica: uma evolução inesperada
Patrícia Queirósa, Diamantino Sousaa, Artur Antunesa, Mercedez Sanchezb, Ricardo Françab, José Casquilhob, Horácio Guerreiroa
aGastroenterology Department, Centro Hospitalar Universitário do Algarve, Faro, Portugal; bSurgery Department, Centro Hospitalar Universitário do Algarve, Faro, Portugal
* Corresponding author.
ABSTRACT
Gastrostomy site metastization is considered an uncommon complication of percutaneous endoscopic gastrostomy (PEG) placement in patients with head and neck tumours, but it is important to consider this possibility when evaluating gastrostomy-related symptoms. The authors present the case of a 40-year-old male with excessive alcohol consumption and active smoking, diagnosed with a stage IV oropharyngeal squamous cell carcinoma. The patient developed a paraneoplastic demyelinating motor polyneuropathy that, associated with tumour mass effect, caused dysphagia with need for nasogastric tube feeding. Treatment with radiotherapy and then chemoradiotherapy was administered and a PEG was placed with the pull method. Cancer remission and resolution of polyneuropathy was achieved, so PEG was removed. Two weeks later, the patient presented with pain and swelling at the gastrostomy site suggesting a local abscess, with improvement after drainage and antibiotic therapy. After 1 month, there was a tumour mass at the gastrostomy site and an oropharyngeal cancer metastasis was diagnosed. The patient underwent surgical excision of abdominal wall metastasis and abdominal disease was controlled. Nevertheless, there was subsequent oropharyngeal neoplasia recurrence and the patient died 6 months later. This case raises the discussion about gastrostomy placement methods that could avoid gastrostomy site metastization, the possible differential diagnosis, and diagnostic workout. Surgical resection may allow metastatic disease control, but by primary disease evolution greatly affects prognosis.
Keywords: Percutaneous endoscopic gastrostomy, Metastasis, Head and neck cancer
RESUMO
A metastização do local de gastrostomia é considerada uma complicação incomum da colocação de gastrostomia endoscópica percutânea (PEG) em pacientes com tumores da cabeça e pescoço, no entanto é importante considerar essa possibilidade ao avaliar sintomas relacionados com a gastrostomia. Os autores apresentam o caso de um homem de 40 anos com consumo excessivo de álcool e tabagismo ativo, diagnosticado com carcinoma espinocelular orofaríngeo no estádio IV. O paciente desenvolveu uma polineuropatia motora desmielinizante paraneoplásica que, associada ao efeito de massa tumoral, causou disfagia com necessidade de alimentação por sonda nasogástrica. Foi administrado tratamento com radioterapia, seguido de quimioradioterapia e foi colocada PEG com o método de pull. Foi obtida remissão tumoral e resolução da polineuropatia, sendo removida a PEG. Duas semanas depois, o paciente apresentou dor e edema no local da gastrostomia, sugerindo um abscesso local, com melhoria após drenagem e antibioterapia. Um mês depois o local da gastrostomia apresentava uma massa tumoral e foi diagnosticada uma metástase do cancro orofaríngeo. O paciente foi submetido a excisão cirúrgica da metástase da parede abdominal, com controlo da doença abdominal. Contudo, houve recorrência neoplásica orofaríngea subsequente e o paciente faleceu 6 meses depois. Este caso levanta a discussão sobre os métodos de realização de gastrostomia que poderiam evitar a metastização do local de gastrostomia, possíveis diagnósticos diferenciais e marcha diagnóstica. A ressecção cirúrgica pode permitir o controle da doença metastática, no entanto o prognóstico é muito afetado pela evolução da doença primária.
Palavras-Chave: Gastrostomia endoscópica percutânea, Metástases, Cancro da cabeça e pescoço
Introduction
Patients with head and neck cancer (HNC) are at high risk of malnutrition. Dysphagia is frequent, related to cancer mass effect and associated systemic disease, and also with treatment adverse effects. A percutaneous endoscopic gastrostomy (PEG) allows long-term enteral nutrition in patients with swallow impairment and may help to avoid deterioration of nutritional status [1, 2]. Gauderer et al. [3] described in 1980 the pull method of gastrostomy tube placement, using endoscopy instead of surgery, being nowadays the most commonly used method in clinical practice, because of its simplicity and safety.
A possible complication of PEG placement in patients with HNC is gastrostomy site metastization, but it is considered uncommon [4]. The authors report a case of gastrostomy site metastization in a patient with HNC, the diagnostic workup, treatment, and outcome.
Clinical Case
A 40-year-old man with an history of excessive alcohol consumption (abstinent for about 1 year) and active smoking, with no other medical or surgical relevant background, was referred to the hospital for evaluation of a mass at the base of the tongue, with 5 months of evolution. He complained of limited tongue movement and progressive dysphagia. Mass biopsies were taken, and histology revealed a squamous cell carcinoma (SCC). The computed tomography (CT) scan showed an oropharyngeal cancer with 60 mm of maximum diameter, at stage IVa (T4aN2M0). Meanwhile, the patient developed a paraneoplastic demyelinating motor polyneuropathy, with respiratory failure and need of invasive ventilation. Neuropathy associated with tumour mass effect caused dysphagia and nasogastric tube feeding was initiated. Given the poor clinical condition it was decided to start palliative radiotherapy, and 30 Gy (3 Gy/cycle/day) were administered. There was great tumour mass reduction and clinical improvement, allowing the start of chemoradiotherapy (CRT) with curative intention, with weekly cisplatin (40 mg/m2) and reaching a total radiotherapy dose of 70 Gy. Because of long-standing nasogastric tube feeding with associated dysphagia and odynophagia, a PEG was placed with the pull method, during the CRT protocol. Removal of the nasogastric tube led to improvement of the dysphagia and oral feeding was initiated. There was complete resolution of polyneuropathy symptoms and 2 months after the end of CRT we observed cancer remission.
Two months later the patient was asymptomatic and desired to remove the PEG. He had a good nutritional status, there were no tumour or inflammatory signs at gastrostomy site, and PEG was removed.
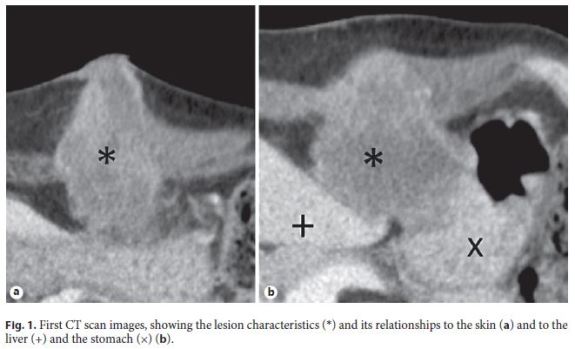
Two weeks after PEG removal, the patient presented pain, swelling, and erythema at the gastrostomy site. CT findings suggested an infectious collection, with 63 mm of maximum diameter (Fig. 1). It was admitted that there was an abnormal closure of the gastrostomy path with abscess formation, so purulent content was drained and antibiotic therapy was started. A biopsy was taken because of the possibility of metastatic disease as a differential diagnosis.
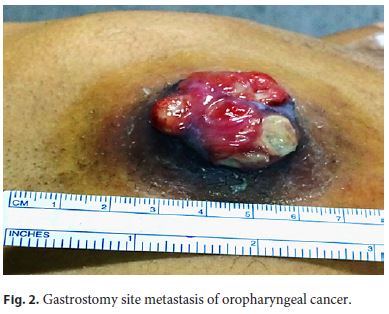
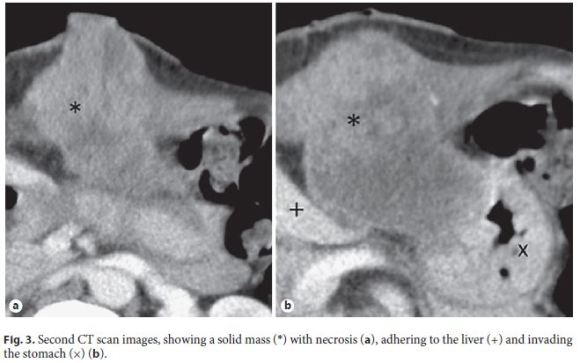
Although there was initial improvement of the inflammatory signs, 1 month later, the patient complained of local pain, nausea, and vomiting, and the gastrostomy site presented a nodular and irregular growth with 3 cm in diameter (Fig. 2). The CT scan showed a solid lesion at the gastrostomy path, with some heterogeneous liquid content, with 84 mm of maximum diameter (Fig. 3), suggestive of neoplastic mass with necrotic content. Previous biopsy revealed an SCC and the diagnosis of gastrostomy metastasis of the oropharyngeal cancer was made. There was a bulging anterior gastric wall in the upper endoscopy and biopsies showed invasion by SCC.
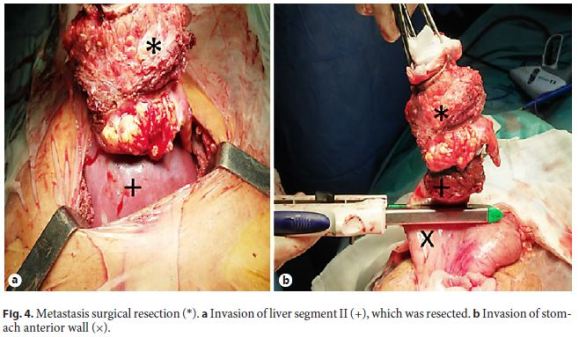
The patient underwent surgical excision of the abdominal wall metastasis, with curative intention, including a portion of the gastric wall and the hepatic border, which presented neoplastic invasion (Fig. 4). Histopathology described a moderately differentiated SCC with extensive areas of necrosis and surgical resection margins free of neoplasia (R0 resection).
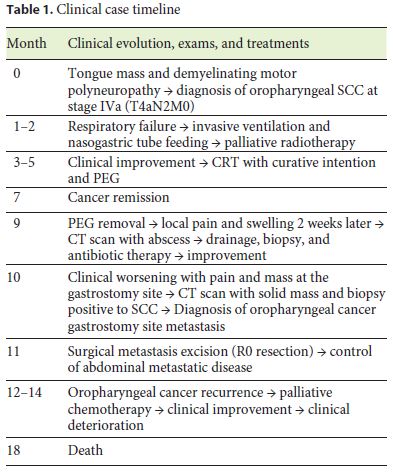
Despite the abdominal disease control there was subsequent oropharyngeal recurrence. Palliative chemotherapy with weekly paclitaxel (75 mg/m2) was started, initially with good response. The patient had 8 weeks of chemotherapy, but then there was a deterioration of his clinical condition, and he died 6 months after the local recurrence (Table 1).
Discussion
PEG tube placement is a frequent procedure in clinical practice, but gastrostomy site metastasis in patients with oesophageal or HNC is considered an uncommon complication, with an estimated frequency between 0.5 and 1% [4]. The first report of gastrostomy site metastasis after PEG was in 1989 [5] and since then the knowledge about this complication is based in case series and case reports. A review of 2013 reported 45 cases described in case series [6], but there are more recent case reports [7, 8].
A recent study analysed 777 patients with HNC following PEG tube placement, detecting five cases of gastrostomy metastasis, with an overall incidence of 0.64%, all of them with PEG tube insertion via a pull method [9]. There are different theories of the pathogenesis of tumour spread to the gastrostomy site, this study supports the theory of direct inoculation of tumour cells at time of tube placement. Cappell [4] identified in 2007 some cancer-related risk factors for gastrostomy site metastization: pharyngoesophageal location, squamous cell histology, advanced cancer stage, poorly differentiated cancer, and large mass size. Most of these risk factors were present in our patient. To avoid this complication other gastrostomy tube placement methods, with no contact with the neoplastic lesion, could be used, like the push method [10], the use of a flexible overtube [11], or radiology-assisted gastrostomy [12].
However, there are other theories of the pathogenesis of tumour spread to the gastrostomy site that consider the possibility of tumour desquamation into the alimentary tract with seeding of the gastrostomy site, and also haematogenous dissemination with deposit of circulating tumour cells in the inflammatory tissue associated with the gastrostomy site [6, 13].
Our patient underwent surgical excision of the gastrostomy site metastasis achieving abdominal disease control, but had an unfavourable outcome because of oropharyngeal recurrence. When surgery is not an option, treatments like radiotherapy may be considered, allowing disease control [9].
The authors consider that, although gastrostomy site metastasis after PEG placement in HNC is considered an uncommon complication, risk factors and local expertise should guide the choice of the methods for gastrostomy tube placement. Gastrostomy-related symptoms should be investigated, considering biopsy to exclude or confirm the metastasis diagnosis. Finally, gastrostomy metastasis does not imply palliative therapy, local and systemic disease must be revaluated, and curative surgical recession of isolated abdominal metastasis may be considered, along with other therapies that may reduce progression of the disease.
References
1 Arends J, Bachmann P, Baracos V, et al: ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [ Links ]
2 Löser C, Aschl G, Hébuterne X, et al: ESPEN guidelines on artificial enteral nutrition – percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005;24:848–861. [ Links ]
3 Gauderer MWL, Ponsky JL, Izant RJ: Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980;15:872–875. [ Links ]
4 Cappell MS: Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: a review of all 44 known reported cases. Am J Gastroenterol 2007;102:1307–1311. [ Links ]
5 Preyer S, Thul P: Gastric metastasis of squamous cell carcinoma of the head and neck after percutaneous endoscopic gastrostomy – report of a case. Endoscopy 1989;21:295. [ Links ]
6 Sinapi I, Navez B, Hamoir M, et al: Seeding of the percutaneous endoscopic gastrostomy site from head and neck carcinoma: case report and review of the literature. Head Neck 2013;35:E209–E212. [ Links ]
7 Fonseca J, Adriana C, Fróis-Borges M, Meira T, Oliveira G, Santos JC: Ostomy metastasis after pull endoscopic gastrostomy: a unique favourable outcome. Nutr Hosp 2015;31:1879–1881. [ Links ]
8 Sousa AL, Sousa D, Velasco F, Açucena F, Lopes A, Guerreiro H: Rare complication of percutaneous endoscopic gastrostomy: ostomy metastasis of esophageal carcinoma. World J Gastrointest Oncol 2013;5:204. [ Links ]
9 Fung E, Strosberg DS, Jones EL, et al: Incidence of abdominal wall metastases following percutaneous endoscopic gastrostomy placement in patients with head and neck cancer. Surg Endosc 2017;31:3623–3627. [ Links ]
10 Russell TR, Brotman M, Norris F: Percutaneous gastrostomy. A new simplified and costeffective technique. Am J Surg 1984;148:132–137. [ Links ]
11 Musumba CO, Hsu J, Ahlenstiel G, et al: Feasibility and safety of overtubes for PEG-tube placement in patients with head and neck cancer. Eur Arch Oto-Rhino-Laryngology 2013;274:3971–3976. [ Links ]
12 Given MF, Lyon SM, Lee MJ: The role of the interventional radiologist in enteral alimentation. Eur Radiol 2004;14:38–47. [ Links ]
13 Brown MC: Cancer metastasis at percutaneous endoscopic gastrostomy stomata is related to the hematogenous or lymphatic spread of circulating tumor cells. Am J Gastroenterol 2000;95:3288–3291. [ Links ]
Statement of Ethics
The information in this paper is presented after the informed consent of the patient was obtained, and respects the patient’s confidentiality.
Disclosure Statement
The authors declare that they have no conflicts of interest.
* Corresponding author.
Dr. Patrícia Queirós
Serviço de Gastrenterologia, Centro Hospitalar Universitário do Algarve
Unidade Hospitalar de Portimão, Sítio do Poço Seco
PT–8500-338 Portimão (Portugal)
E-Mail patriciaqueiros1@hotmail.com
Received: December 12, 2017; Accepted after revision: January 18, 2018
Author Contributions
Patrícia Queirós and Diamantino Sousa contributed equally to this work; Patrícia Queirós and Diamantino Sousa designed and wrote the article; Artur Antunes placed the percutaneous endoscopic gastrostomy and critically revised the article; Mercedez Sanchez, Ricardo França, and José Casquilho were the surgical team and critically revised the article; Horácio Guerreiro critically revised the article. All the authors approved the final version to be published.














