Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.3 Lisboa jun. 2019
https://doi.org/10.1159/000491611
ORIGINAL ARTICLE
Variation between Pathological Measurement and Endoscopically Estimated Size of Colonic Polyps
Variação entre a medição patológica e o tamanho endoscópico estimado de pólipos do cólon
Catarina Atalaia-Martins, Pedro Marcos, Carina Leal, Sandra Barbeiro, Alexandra Fernandes, Antonieta Santos, Liliana Eliseu, Cláudia Gonçalves, Isabel Cotrim, Helena Vasconcelos
Gastroenterology Department, Centro Hospitalar de Leiria, Leiria, Portugal
* Corresponding author.
ABSTRACT
Background and Aims: Accurate determination of colonic polyp size is vital to an appropriate surveillance. The main aim of this study was to evaluate variation between the polyp size reported by the endoscopist and its pathological measurement. Methods: A retrospective analysis of all colonic adenomatous polyps resected in a 12-month period was performed at our center. Endoscopic and pathological size for each polyp were compared, and overestimation rates, underestimation rates, and endoscopic-pathological variation (EPV) were calculated. Results: Among the 573 polyps that were included, the mean endoscopic and pathological sizes were 8.00 and 6.66 mm, respectively. The most frequent error, in 62.1%, was overestimation by the colonoscopist. Overestimation and EPV were associated with resection technique (higher in endoscopic mucosal resection and smaller with biopsy forceps) and colonoscopist. They were not associated with years of experience in colonoscopy. Overestimation was more frequent in larger polyps. Conclusions: Our study shows significant discordance between endoscopic and pathological size of colonic polyps with a clear tendency for endoscopic overestimation. Larger polyps are more difficult to accurately assess than smaller ones. This propensity for error was not related to colonoscopist’s years of experience and seems to be an individual tendency.
Keywords: Colonic polyps, Polyp size, Polyp surveillance
RESUMO
Introdução e objetivos: A precisão na determinação do tamanho de pólipos do cólon é vital para uma vigilância adequada. O objetivo deste trabalho foi avaliar a variação entre o tamanho reportado pelo endoscopista e pelo anatomo-patologista. Métodos: Foi realizada uma análise retrospetiva de todos os pólipos adenomatosos ressecados, num período de 12 meses, no nosso centro. O tamanho endoscópico e patológico de cada pólipo foi comparado e foram calculadas as taxas de sobrestimativa, subestimativa e a variação endoscópica-patológica (VEP). Resultados: Foram incluídos 573 pólipos, tamanho endoscópico e patológico médio de 8,00 e 6,66 milímetros, respetivamente. O erro mais frequente, em 62.1% foi a sobrestimativa pelo endoscopista. A sobrestimativa e a VEP associaram-se à técnica de resseção (maior na resseção endoscópica da mucosa e mais pequena na pinça de biópsias) e ao colonoscopista. Não se associaram aos anos de experiência em colonoscopia. A sobrestimativa foi mais frequente nos pólipos maiores. Conclusões: O nosso trabalho mostrou uma discordância significativa entre o tamanho endoscópico e patológico de pólipos do cólon com uma clara tendência para a sobrestimativa. Os pólipos maiores são mais difíceis de avaliar com precisão do que os mais pequenos. Esta propensão para o erro não se relacionou com os anos de experiência em colonoscopia e parece ser uma tendência individual.
Palavras-Chave: Pólipos do colon, Tamanho de pólipos, Vigilância de pólipos
Introduction
Screening of colorectal cancer (CRC) in asymptomatic patients can decrease both incidence and mortality of CRC [1]. Colonoscopy enables the identification and removal of premalignant polyps [2], and close to 20% of endoscopic procedures are surveillance colonoscopies [3]. Surveillance guidelines are based on risk stratification predicted by findings on index colonoscopy and allocate patients to 2 groups: low and high risk of developing advanced adenomas. The high-risk group includes patients with polyps with villous histology, high-grade dysplasia, 3 or more adenomas, or at least 1 adenoma with 10 mm or more [1, 4].
Determination of polyp size is vital to the assessment of CRC risk and surveillance [5]. Inaccurate size estimation may lead to the performance of unnecessary procedures [6] or misdirect patients to less aggressive followup [5]. There are several studies showing significant inaccuracy of endoscopically estimated polyp size when compared with pathological size [6–8]. An overestimation or underestimation is more likely to be important when the misjudgment crosses the 10-mm threshold [9].
Inappropriate surveillance recommendations due to poor estimates range from 10% [6] to 47.8% [7]. Even after considering histological features and synchronous polyps [7], inappropriate surveillance rates remain high. Endoscopic size results from a subjective 2-dimensional visual estimation [10] and has considerable variability among colonoscopists [6, 8, 9]. It is influenced by several factors, including colonoscopist’s terminal digit preference [11] and distortion effect of fish eye lenses: objects located at the center of the displayed view appear magnified compared to objects located at the periphery [12]. Pathological size results from measurement with a standard ruler, after resection, directly measured either as a fresh specimen or after fixation in formalin [13]. All measurements should be reported to the nearest mm and not rounded [14]. Pathological size is more accurate and should be used to determine surveillance [9, 15]. However, an accurate endoscopic estimation of size remains crucial because there are still some situations where endoscopic size should be used, for example when pathological size is not available or if the lesion is fragmented [14]. Additionally, to determine appropriate surveillance intervals, an accurate endoscopic size is also crucial for safe implementation of optical diagnosis [12] for the “do not resect” and “resect and discard” strategies [16].
Although pathological size is the preferred method to assess size, there are also several factors that can affect it, namely handling of specimens that may cause distortion of the polyp, effect of coagulation during polypectomy [12], vascular collapse [17], formalin fixation effect, and (like the colonoscopist’s) the histopathologist’s terminal digit preference of 0 or 5 [11].
The accuracy of endoscopic size measurements should be monitored, deviation between pathologists and endoscopists should be minimized, and management decisions dependent on size should consider potential inaccuracy in the size measurement [15]. The main aim of this study was to evaluate variation between colonic polyp size reported by the endoscopist and its pathological measurement. The secondary aim was to determine if variables related to the polyp (polyp morphology or resection technique) or to the colonoscopist (executant or years of experience in colonoscopy) were associated with inaccuracy of endoscopic size.
Methods
This was a retrospective observational unicentric study. All colonoscopies performed in a 12-month period by 7 experienced colonoscopists at our center were assessed. All adenomatous pol yps resected in a single specimen, not fragmented during retrieval, and with precise documentation of endoscopically estimated size, resection technique, and pathological measurement were included. Colonoscopies were performed using the colonoscopes Olympus® CF-Q165L and CF-H180AL (Olympus, Tokyo, Japan). Endoscopic sizes, polyp morphology, and resection techniques were collected from colonoscopy reports. Helper tools to estimate the endoscopic size were not used. Pathological measurement was obtained after fixation with formalin (10% formalin solution) measured from the slide, unless the polyp was too big; in that case, it was measured from the fixed specimen at the time of dissection. Pathological sizes were collected from pathology reports. Polyps greater than 25 mm; polyps with unclear size (not reported or reported imprecisely); more than 1 polyp placed in the same container after retrieval, and patients with inflammatory bowel disease or a history of CRC were excluded. The Paris Classification was used for polyp morphology. Colonoscopies were performed by skilled colonoscopists, whose years of experience ranged from 4 to 25 years.
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS), version 22 (IBM, Armonk, NY, USA). Descriptive statistics were used for the outcome of interest. All endoscopic and respective pathological sizes were compared. To assess accuracy, overestimation, and underestimation rates and endoscopic-pathological variation (EPV; defined by the difference between endoscopic size and pathological size) were calculated. Statistical tests used were one-way ANOVA, χ2 test, and Spearman’s correlation coefficient. The significance level to reject the null hypothesis was considered to be ≤0.05.
Results
A total of 3,997 colonoscopies were assessed, and 573 polyps were included, corresponding to 507 patients. Among these, 58.3% were male; patients had a mean age of 64.8 ± 11.9 years. The majority of the polyps had a sessile morphology, and hot snare polypectomy was the most frequently used resection technique. Table 1 summarizes the polyp morphology and resection techniques. The mean endoscopic size was 8.00 ± 5.33 mm, and the mean pathological size was 6.66 ± 4.87 mm. The mean years of experience in colonoscopy was 13. The most frequent error, in 62.1%, was overestimation of the polyp size by the endoscopist when compared with pathological size. In only 26.2% of the cases, the endoscopic and pathological size were matched, and in 11.7%, the endoscopist underestimated polyp size.
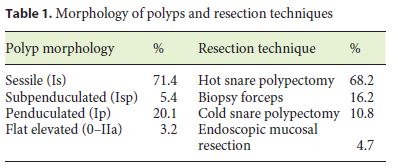
The endoscopic overestimation was significantly associated with resection technique (χ2 (3) = 9.363, p = 0.025) and executant (χ2 (6) = 22.729, p < 0.001) and not associated with polyp morphology (p = 0.122) and years of experience in colonoscopy (p = 0.181). The overestimation rate was significantly higher in endoscopic mucosal resection and lower in the biopsy forceps group. The individual overestimation rate was not similar among all colonoscopists, varying from 39.2 to 74.3%. Figure 1 shows the accuracy of endoscopic size per endoscopist.
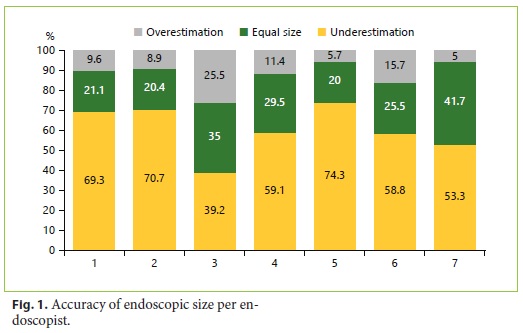
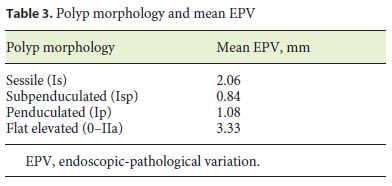
The mean EPV was 1.34 mm, and it was significantly associated with polyp morphology (F(3, 51.969) = 8.490, p < 0.001), resection technique (F(3, 93.399) = 10.733, p < 0.001), and executant (F(6, 195.871) = 5.928, p < 0.001) and not associated with years of experience in colonoscopy (p = 0.280). Table 2 shows the endoscopic overestimation rate and EPV in different resection techniques. The EPV was significantly higher in flat elevated (IIa) polyps, as illustrated in Table 3, and significan- tly higher in endoscopic mucosal resection. EPV was significantly different between colonoscopists, varying from 0.25 to 2 mm.
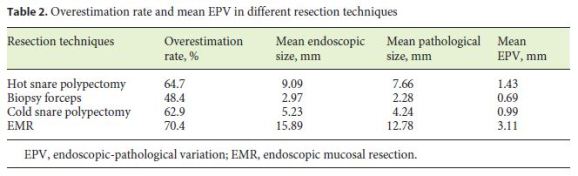
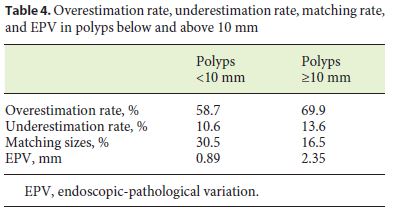
Polyps were divided into 2 groups: polyps up to 10 mm and polyps with 10 mm or more, and overestimation rate and EPV were assessed. The overestimation was higher in polyps with 10 mm or more (69.9 vs. 58.7%), as was the mean EPV (2.35 vs. 0.89 mm). A match of the sizes was more frequent in the smaller-polyps group (30.5 vs. 16.5%). Table 4 shows the overestimation rate, underestimation rate, matching rate, and EPV in each of the groups.
Discussion
Endoscopic and pathological sizes are both subject to variability [12]. Despite the recommendation of the use of pathological size to determine surveillance, in clinical practice sometimes the endoscopic size is the only measurement available as in the case of resections performed in a piecemeal fashion, fragmentation during retrieval, or when pathological size is not available [15]. Thus, an accurate endoscopic estimation is crucial. Our study shows significant discordance between endoscopic and pathological size of colonic polyps with a clear tendency to endoscopic overestimation. Results from other studies are in agreement with ours, showing endoscopic overestimation in most cases of poor estimation [5–8, 17, 18]. Additionally, some studies showed opposite results with a tendency to endoscopic underestimation [13, 19–21], but almost all of them used artificial colon models [19–21] which may not reflect the same in vivo conditions. In our study, polyps resected by endoscopic mucosal resection were more frequently overestimated and had a higher EPV, and the inverse was observed for polyps resected with biopsy forceps. This may suggest that larger polyps are more difficult to accurately assess than smaller ones. We also confirmed this hypothesis by a subanalysis by size, showing that polyps larger than 10 mm had a higher overestimation rate and a higher EPV than polyps smaller than 10 mm. Although a comparison of the polyp with an open biopsy forceps is not routinely used to assess size, we speculate that its opening, prior to resection, may contribute to an improved size estimation.
Our study only included adenomatous polyps, but the influence of polyp histology on accuracy remains controversial. While Schoen et al. [8] showed that inaccuracy was not dependent on histology (classifying polyps only into 2 groups: adenomatous or nonadenomatous), Anderson et al. [5] concluded that histology type did not influence overestimation rates, despite serrated polyps (vs. adenomas and hyperplastic polyps) conferring higher underestimation rates. In our study, the EPV was significantly associated with flat elevated morphology. This finding can possibly be explained by the borders of flat lesions which are frequently more difficult to distinguish than other morphology types. Anderson et al. [5] also concluded that nonpedunculated polyp configuration (sessile or flat appearance) was a significant risk factor for endoscopic overestimation, but most of the studies do not address polyp morphology.
Overestimation rate and EPV varied significantly among colonoscopists but are not related with years of experience, probably reflecting an individual tendency. This lack of association with years of experience is also supported by other authors [5, 13]. Some techniques have been described to reduce colonoscopists’ estimation error. One of the most widespread techniques is the comparison of the polyp with an open biopsy forceps with a known diameter which is held against it. But, besides being time-consuming, some authors showed that there is still a great interobserver variability [6, 10, 19]. Several other devices to minimize endoscopists’ error have been described, such as a graduated ruler [22], a calibrated hood for use in cap-assisted colonoscopy [23, 24], a linear probe [25], and a virtual tape measure containing a laserline emitter [26]. Some of the devices described are prototypes, used only in investigation settings and tested in artificial or animal models, and others are not widely available or were not generally adopted in clinical practice. Thus, there is no standardized technique to improve endoscopic size estimation.
Our study has some strengths that must be highlighted. Despite the retrospective design, which is a limitation potentially leading to bias, it enabled us to retrieve data of real-life and daily routine practice. Also, unlike most studies in this research area, we were able to gather a large sample size, and, to the best of our knowledge, there is only 1 study with a larger cohort than ours. Additionally, we included polyps with all sizes up to 25 mm, while some studies excluded small polyps. Our study also has some limitations. First, pre- and postfixation measurements were not compared. However, several studies have refuted the previous belief that formalin shrinks the polyp, demonstrating no significant differences between fresh and fixed specimens [8, 13, 17], so it seems unlikely that the size difference is justifiable by formalin effect. Second, the retrieval method which may influence polyp size was not recorded due to insufficient data from colonoscopy reports. Finally, we cannot evaluate the impact on surveillance intervals because we did not take into account other variables, such as total number of adenomas, histologic features, or bowel preparation.
In conclusion, our study demonstrates a significant inaccuracy of endoscopically estimated size with a propensity for overestimation. Our results suggest a clear need for improvement and raise questions about inappropriate surveillance intervals with all the costs and risks associated with unnecessary colonoscopies. Being aware of our own limitations is an important step towards improvement. Are the colonoscopists aware of their own tendency for error? Would a regular monitoring of discrepancies in endoscopically estimated sizes contribute to improving accuracy? Will technology be able to provide widely available new tools? Further investigation is needed to address these questions.
References
1 Lieberman DA, Rex DK, Winawer SJ, et al: Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2012;130:1872–1885. [ Links ]
2 Young PE, Womeldorph CM: Colonoscopy for colorectal cancer screening. J Cancer 2013;4:217–226. [ Links ]
3 Lieberman DA, Williams JL, Holub JL, et al: Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc 2014;80:133–143. [ Links ]
4 Hassan C, Quintero E, Dumonceau J-M, et al: Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013;45:842–851. [ Links ]
5 Anderson BW, Smyrk TC, Anderson KS, et al: Endoscopic overestimation of colorectal polyp size. Gastrointest Endosc 2016;83:201–208. [ Links ]
6 Chaptini L, Chaaya A, Depalma F, Hunter K, Peikin S, Laine L: Variation in polyp size estimation among endoscopists and impact on surveillance intervals. Gastrointest Endosc 2014;80:652–659. [ Links ]
7 Eichenseer PJ, Dhanekula R, Jakate S, Mobarhan S, Melson JE: Endoscopic mis-sizing of polyps changes colorectal cancer surveillance recommendations. Dis Colon Rectum 2013;56:315–321. [ Links ]
8 Schoen RE, Gerber LD, Margulies C: The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc 1997;46:492–496. [ Links ]
9 National Cancer Screening Service: Guidelines for Quality Assurance in Colorectal Screening, ed 1. 2012. https://www.screeningservice.ie/publications/Guidelines-for-Quality-Assurance-in-Colorectal-Screening.pdf. [ Links ]
10 Rex DK, Rabinovitz R: Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc 2014;79:402–407. [ Links ]
11 Plumb AA, Nickerson C, Wooldrage K, et al: Terminal digit preference biases polyp size measurements at endoscopy, computed tomographic colonography, and histopathology. Endoscopy 2016;48:899–908. [ Links ]
12 Vleugels J LA, Dekker E: Does polyp size matter? Endosc Int Open 2017;5:746–748. [ Links ]
13 Moug SJ, Vernall N, Saldanha J, Mcgregor JR, Balsitis M, Diament RH: Endoscopists’ estimation of size should not determine surveillance of colonic polyps. Color Dis 2010;12:646–650.
14 Atkin WS, Valori R, Kuipers EJ, et al: European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition – colonoscopic surveillance following adenoma removal. Endoscopy 2012;44(suppl 3):151–163. [ Links ]
15 Quirke P, Risio M, Lambert R, von Karsa L, Vieth M; International Agency for Research on Cancer: European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition – quality assurance in pathology in colorectal cancer screening and diagnosis. Endoscopy 2012;44:SE116–SE130. [ Links ]
16 Ferlitsch M, Moss A, Hassan C, et al: Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2017;49:270–297. [ Links ]
17 Morales TG, Sampliner RE, Garewal HS, Fennerty MB, Aickin M: The difference in colon polyp size before and after removal. Gastrointest Endosc 1996;43:25–28. [ Links ]
18 Izzy M, Virk MA, Saund A, Tejada J, Kargoli F, Anand S: Accuracy of endoscopists’ estimate of polyp size: A continuous dilemma. World J Gastrointest Endosc 2015;7:824–829.
19 Margulies C, Krevsky B, Catalano MF: How accurate are endoscopic estimates of size? Gastrointest Endosc 1994;40:174–176. [ Links ]
20 Fennerty MB, Davidson J, Emerson SS, Sampliner RE, Hixson LJ, Garewal HS: Are endoscopic measurements of colonic polyps reliable? Am J Gastroenterol 1993;88:496–500. [ Links ]
21 Rubio CA, Höög CM, Broström O, et al: Assessing the size of polyp phantoms in tandem colonoscopies. Anticancer Res 2009;29:1539–1545. [ Links ]
22 Kaz AM, Anwar A, O’Neill DR, Dominitz JA: Use of a novel polyp “ruler snare” improves estimation of colon polyp size. Gastrointest Endosc 2016;83:812–816.
23 Watanabe T, Kume K, Yoshikawa I, Harada M: Usefulness of a novel calibrated hood to determine indications for colon polypectomy: visual estimation of polyp size is not accurate. Int J Colorectal Dis 2015;30:933–938. [ Links ]
24 Kume K, Watanabe T, Yoshikawa I, Harada M: Endoscopic measurement of polyp size using a novel calibrated hood. Gastroenterol Res Pract 2014;2014:714294. [ Links ]
25 Gopalswamy N, Shenoy VN, Choudhry U, et al: Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc 1997;46:497–502. [ Links ]
26 Goldstein O, Segol O, Siersema PD, Jacob H, Gross SA: Novel device for measuring polyp size: an ex vivo animal study. Gut 2017, DOI: 10.1136/gutjnl-2017-314829. [ Links ]
Statement of Ethics
The local ethics committee approved this study.
Disclosure Statement
The authors have no competing interests to declare.
* Corresponding author.
Dr. Catarina Atalaia-Martins
Department of Gastroenterology, Centro Hospitalar de Leiria
Rua das Olhalvas
PT–2410-197 Leiria (Portugal)
E-Mail catarinatalaiamartins@gmail.com
Received: March 5, 2018; Accepted after revision: June 15, 2018
Acknowledgements
The authors would like to thank Dr. Nuno Vicente Rodrigues for constructive criticism of the manuscript.
Author Contributions
Catarina Atalaia-Martins designed the study, drafted the article, and is the article guarantor. Pedro Marcos, Carina Leal, and Sandra Barbeiro analyzed the data. Alexandra Fernandes, Antonieta Santos, Liliana Eliseu, Cláudia Gonçalves, and Isabel Cotrim critically reviewed the article for intellectual content. Helena Vasconcelos approved the article.














