Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.4 Lisboa ago. 2019
https://doi.org/10.1159/000492066
CLINICAL CASE STUDY
Improvement of Central Nervous System Vasculitis in a Patient with Chronic Hepatitis C Virus Infection after Treatment with an Interferon-Free Regimen
Melhoria de vasculite do sistema nervoso central em doente com hepatite C após tratamento com antivíricos de ação direta
Sérgio Limaa, Raquel Fariaa,b, Filipe Neryc–e
aServiço de Medicina Interna, Centro Hospitalar do Porto, Hospital de Santo António, Porto, Portugal; bUnidade de Imunologia Clínica, Centro Hospitalar do Porto, Hospital de Santo António, Porto, Portugal; cUnidade de Transplante Hepato-Pancreática, Centro Hospitalar do Porto, Hospital Santo António, UTHP, Porto, Portugal; dEPIUnit – Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal; eICBAS – Instituto de Ciências Biomédicas de Abel Salazar, Universidade do Porto, Porto, Portugal
* Corresponding author.
ABSTRACT
Background: Neurosarcoidosis is a rare manifestation of hepatitis C virus (HCV) infection, mainly in patients exposed to interferon-based therapies. Although we are living in a new era of HCV treatment, there is still little data concerning the treatment of extrahepatic complications of the disease with direct antiviral agents, especially rare ones such as neurosarcoidosis. Summary: We present a rare case of central nervous system vasculitic lesions in the context of chronic HCV infection associated with mixed cryoglobulinemia, elevated angiotensin-converting enzyme (ACE) levels, and documentation of viral RNA in the cerebrospinal fluid in a treatment-naïve chronic HCV patient. Successful treatment with an interferon-free regimen improved all clinical manifestations, reduced the levels of serum ACE, and reduced the cryoglobulin levels to undetectable. Messages: Neurosarcoidosis and cryoglobulinemia are rare but well-recognized complications of HCV infection, even in treatment-naïve patients. Direct antiviral agents can be useful in the management of this condition.
Keywords: Hepatitis C virus infection, Neurosarcoidosis, Central nervous system vasculitis, Direct antiviral agents, Cryoglobulinemia
RESUMO
Introdução: A neurossarcoidose é uma manifestação rara da infeção por hepatite C, sobretudo associada a exposição prévia a esquemas com interferão. Apesar de estarmos numa nova era do tratamento da hepatite C, há ainda poucos dados em relação ao papel dos antivíricos de ação direta no tratamento das complicações extrahepáticas da doença, especialmente as raras, como a neurossarcoidose. Sumário: Apresentamos um caso raro de vasculite do Sistema nervoso central associada a elevação dos níveis de enzima conversora da angiotensina, documentação de RNA viral no líquido cefalo-raquidiano num doente com infeção crónica a hepatite C sem tratamentos prévios. O tratamento com antivíricos de ação direta melhorou todas as manifestações clínicas, incluindo redução dos níveis de ECA e de crioglobulinas. Mensagens: A neurossarcoidose é uma rara, mas bem reconhecida complicação da hepatite C, mesmo em doentes sem exposição prévia a interferão. Os agentes de ação direta podem ser úteis na gestão de ambas as doenças.
Palavras-Chave: Hepatite C, Neurossarcoidose, Vasculite do sistema nervoso central, Antivíricos de ação direita, Crioglobulinemia
Introduction
One-hundred and eighty million people worldwide are estimated to have chronic hepatitis C virus (HCV) infection. Liver manifestations vary according to the degree of fibrosis, portal hypertension complications, and the severity of liver failure. Mixed cryoglobulinemia (present in up to 50% of the patients), non-Hodgkin lymphoma, and chronic renal disease are some of the immune complex-mediated manifestations of chronic hepatitis C. In addition, a large spectrum of neuropsychiatric manifestations may be present, probably linked to vascular inflammatory changes in the central nervous system [1]. Sarcoidosis has been associated with chronic HCV infection, but mainly in patients exposed to interferon (IFN)-based therapies [2]. Neurosarcoidosis, which may be present in up to 5% of the patients with systemic sarcoidosis, has rarely been described in HCV-infected patients, only as case reports and usually related to IFN exposure [2]. In the IFN-free regimen era we are currently living in, treating HCV extrahepatic manifestations with direct antiviral agents (DAAs) is recommended [3], leaving other immunosuppressive therapies, such as corticosteroids or rituximab, usually used in combination with DAAs for very severe manifestations. We describe a rare case of a patient diagnosed with neurosarcoidosis and cryoglobulinemia due to HCV infection during the investigation of neuropsychiatric manifestations, whose symptoms completely resolved with DAA treatment and the resolution of HCV infection.
Clinical Case
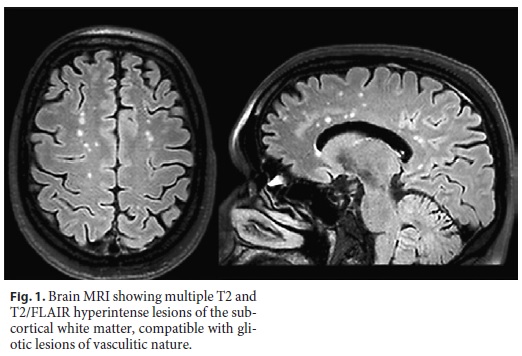
A 45-year-old female was referred to the internal medicine outpatients clinic after the identification of several diffuse gliotic lesions on cerebral magnetic resonance imaging (MRI) and elevated levels of angiotensin-converting enzyme (ACE) in serum and cerebrospinal fluid (CSF). She had a known history of clinical depression and epilepsy not adequately controlled with sodium valproate treatment. These imaginological findings were identified after investigation of peripheral right facial nerve palsy, secondary generalized partial seizures, and finger-nose dysmetria. The initial computed tomography scan revealed several supratentorial hypodensities. Multiple foci of diffuse gliotic lesions in both brain hemispheres suggestive of inflammatory/vasculitic etiology were found on cerebral MRI (Fig. 1). Serum ACE levels were raised (436 UI/mL) and were associated with positive rheumatoid factor (26 UI/mL). CSF analysis showed normal glucose (74 mg/dL) and protein (0.26 g/L) levels, mild pleocytosis (5 leucocytes/mL after correction for traumatic tap), and positive ACE levels (7 UI/mL). These findings were consistent with neurosarcoidosis, although tissue documentation of granulomatous inflammation was not possible.
Diagnosis of a genotype 3a chronic HCV infection was concomitantly made, with a viral load of 568,000 UI/mL. Liver profile showed raised aspartate aminotransferase (.2) and alanine aminotransferase (.2), normal bilirubin (0.36 mg/dL), mild thrombocytopenia (124,000/μL), and an international normalized ratio of 1.02. Bridging fibrosis and moderate activity was found in the liver biopsy (METAVIR score A2F3). The patient tested positive for type II serum cryoglobulins (25 UI/mL). By this time, a second lumbar puncture was performed to determine the hepatitis C viral load in CSF, which was positive (87 UI/mL). In addition, lowerlimb vasculitic lesions developed, interpreted in the context of the above-mentioned cryoglobulinemia.
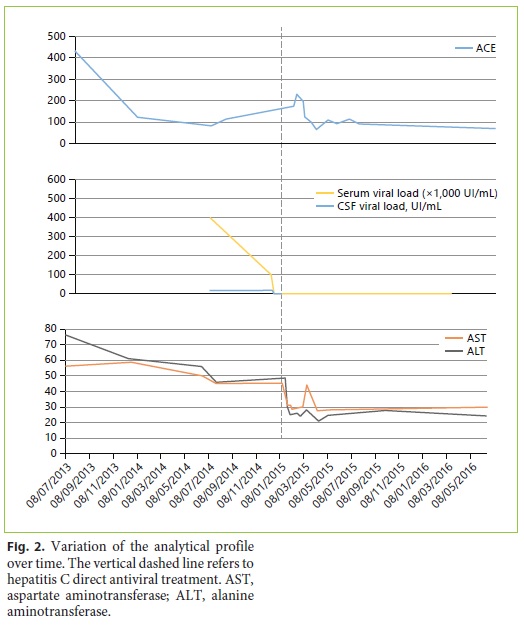
Due to extrahepatic immunologic manifestations of HCV infection (neurosarcoidosis and cryoglobulinemia-associated vasculitis), urgent treatment with an IFN-free regimen was proposed. The patient was started on sofosbuvir 400 mg/day, daclatasvir 90 mg/day, and ribavirin 1,000 mg/day therapy for 24 weeks, according to the European guidelines at that time. Her viral load at week 4 of treatment was already negative with normalization of liver enzymes. Cryogobulinemia resolved, and ACE levels in serum and CSF decreased to 69 and 3 U/L, respectively (Fig. 2). The patient experienced complete resolution of the lower-limb vasculitic lesions and progressive improvement of the facial paresis as well as cerebellar symptoms. Sustained virological response was documented 12 weeks after completion of treatment. A repeated MRI scan (8 months after HCV infection resolution) still demonstrated multiple gliotic lesions but without progression when compared to the first examination. The patients epilepsy is currently controlled with 1,500 mg of sodium valproate/day, with no more partial or generalized crisis documented ever since.
Comment
HCV infection exclusion is mandatory in the suspicion of sarcoidosis and/or vasculitic lesions. On the other hand, central nervous system involvement in HCV chronic infection is a heterogeneous entity. In this particular case, the presence of inflammatory lesions on MRI prompted the evaluation for immunologic manifestations of the disease. The simultaneous identification of elevated serum and CSF ACE levels raised the suspicion of neurosarcoidosis.
Sarcoidosis is a known complication of chronic HCV infection but is mainly recognized as a complication of IFN-based therapies. Reports of the disease in treatmentnaive patients exist but are scarce. The pathogenic mechanism is not fully understood, but the accumulated evidence suggests that this is more than a casual association [2]. The decrease in ACE levels after the successful treatment of HCV infection with an IFN-free regimen reinforces a viral and/or viral-immunological related etiology, as secondary mixed cryoglobulinemia may also have a role in central nervous system lesions. Although peripheral nervous system and cutaneous manifestations are far more common with this entity, the central nervous system may also be involved, particularly with widespread vasculitic lesions, as seen in this patient [4]. Our patient developed peripheral nervous system (peripheral facial nerve palsy) and cutaneous manifestations (lower-limb vasculitis) of the disease, both of which regressed after successful treatment of the HCV infection and cryoglobulin clearance.
Direct central nervous system involvement of HCV infection may be more common than previously suspected. There are several studies demonstrating the presence of the HCV virons both in the CSF and brain tissue of patients with HCV infection [5]. Nonetheless, the link between direct central nervous system infection and the development of vasculitic lesions of the brain is weak. Additionally, the identification of the virus in CSF analysis (as with this patient) may merely reflect viral carriage from the periphery and not active sites of replication [6]. Yet, and importantly, the use of DAAs that may penetrate the blood-brain barrier should probably be considered when choosing a therapeutic scheme to treat neurological manifestations associated with HCV infection, although specific data on this subject is currently lacking.
Conclusion
We present a rare case of central nervous system vasculitic lesions in the context of chronic HCV infection associated with mixed cryoglobulinemia, elevated ACE levels, and documentation of viral RNA in the CSF in a treatment-naive chronic HCV patient. Successful treatment with an IFN-free regimen improved all clinical manifestations, reduced the levels of serum ACE, and reduced the cryoglobulin levels to undetectable. Although there are some alternative explanations, neurosarcoidosis seems to be the best one to account for the clinical picture. This case shows the possible beneficial effects of the new IFN-free regimens in the management of this condition, even before the consideration of other immunosuppressive treatments usually used in this kind of immunological disturbances.
References
1 Monaco S, Ferrari S, Gajofatto A, et al: HCV-related nervous system disorders. Clin Dev Immunol 2012;2012:1–9. [ Links ]
2 Ramos-Casals M, Mana J, Nardi N, et al: Sarcoidosis in patients with chronic hepatitis C infection: analysis of 68 cases. Medicine (Baltimore) 2005;84:69–80. [ Links ]
3 Ramos-Casals M, Zignego A, Ferri C, et al: Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J Hepatol 2017;66:1282–1299. [ Links ]
4 Casato M, Saadoun D, Marchetti A, et al: Central nervous system involvement in hepatitis C virus cryoglobulinemia vasculitis: a multicentre case-control study using magnetic resonance imaging and neuropsychological tests. J Rheumatol 2005;32:484–488. [ Links ]
5 Weissenborn K, Tryc AB, Heeren M, et al: Hepatitis C virus infection and the brain. Metab Brain Dis 2009;24:197–210. [ Links ]
6 Fletcher NF, McKeating JA: Hepatitis C virus and the brain. J Viral Hepat 2012;19:301–306. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Sérgio Lima
Serviço de Medicina Interna, Centro Hospitalar do Porto
Hospital de Santo António, Largo Professor Abel Salazar
PT–4099-001 Porto (Portugal)
E-Mail sergio.lima.fmup@gmail.com
Received: April 29, 2018; Accepted after revision: June 27, 2018














