Introduction
Pancreatic cancer incidence is increasing, and surgery remains the only curative treatment. However, even in specialized centers, the incidence of postoperative complications remains as high as 40-60% with a 5-year survival rate of 10-20% [1].
Operative mortality has improved through surgical technique and perioperative care optimization. In particular, the enhanced recovery after surgery (ERAS) pathway allows a standardized, multimodal, multidisciplinary approach aimed at favoring postoperative recovery by reducing surgical metabolic stress and limiting organ dysfunction [2].
With regard to nutritional status, recent studies have suggested that body composition phenotypes may influence postoperative and long-term clinical outcomes. Most studies have focused primarily on the impact of sarcopenia (low skeletal-muscle mass) on major postoperative complications [3] and overall survival (OS) [1, 4-12], and secondarily on visceral adipose tissue [3, 8, 10, 13] and low muscle radiation attenuation (a marker of fat infiltration of skeletal muscle) [9-11, 13]. Although some of these studies have included body mass index (BMI) [4, 12] as a proxy of body fatness, the influence of skeletal muscle infiltration by adipose tissue as well as the proportion of visceral adipose tissue with regard to skeletal-muscle tissue has been strikingly less studied.
Bearing in mind that obesity is a recognized risk factor for pancreatic cancer and that many of these patients experience weight loss at diagnosis which will certainly translate into a decline of skeletal muscle mass, we hypothesized that all tissues, namely skeletal muscle, visceral fat, and skeletal muscle infiltration by adipose tissue, may be equally relevant. We believe that this approach could lead us to a more comprehensive view where all tissues have a different role but are equally important and expected to interplay. We therefore aimed to study the association of body composition parameters, i.e., skeletal muscle, visceral fat, and muscle radiation attenuation with postoperative complications, 90-day survival, and OS in patients undergoing pancreatic surgery.
Material and Methods
We conducted a single-center retrospective study at the Beatriz Angelo Hospital (HBA). We reviewed all patients undergoing pancreatic surgery at our hospital between March 2012 and December 2017. To be eligible for our study, patients needed to have an abdominal computed tomography (CT) scan performed at our institution within 30 days of surgery to allow body composition analysis.
Demographic and clinical data including age, gender, American Society of Anesthesiologists (ASA) score, disease location, and histology according to surgical specimens were retrieved from patients’ electronic charts. The 90-day mortality and postoperative complications were classified according to Clavien-Dindo classification. We considered the rate of complications as grade I-IIIa versus grade IIIb-V [14]. The date of the last follow-up and death were recorded as well. Primary outcome was OS measured in months from the date of elective hospitalization for surgery until death or until the censor date of the last visit to the hospital. The 90-day survival was recorded in months from the date of elective hospitalization for surgery until death or until the censor date set at 90 days after surgery.
Body Composition Assessment
Weight and reported height were recorded on admission and body mass index (BMI) was computed. BMI classification was done according to the following categories for adults: < 18.5 = underweight, 18.5-24.9 = normal weight, 25.0-29.9 = overweight, 30.0-34.9 = class I obesity, 35-39.9 = class II obesity, and ≥40 = class III obesity. The BMI classification for the elderly was used for patients aged ≥65 years, i.e., < 24 = underweight, 24-27 = normal weight, and > 27 = overweight. Opportunistic body composition assessment was conducted from the diagnostic or staging CT scan. CT methodology is highly precise for quantifying specific tissues and predicting whole-body composition [15]. Images were selected by radiologists on the axial plane at the level of the 3rd lumbar vertebra including both transverse processes using a portal venous phase, and then processed with a program built with Matlab. This software performs an automatic segmentation of tissue cross-sectional areas, using the following Hounsfield unit (HU) thresholds: -29 to 150 for skeletal muscle, -190 to -30 for subcutaneous and intramuscular adipose tissue, and -50 to -150 for visceral adipose tissue. Validation of the processed images was conducted and manual corrections were executed by radiologists. Cross-sectional skeletal muscle, visceral fat, subcutaneous fat, and mean muscle radiation attenuation were recorded. The skeletal muscle index (SMI [in cm2/m2] = skeletal muscle area [SMA]/height) and ratio of visceral fat area/SMA (VFA:SMA) were calculated as previously described [3, 16]. Sarcopenia, low muscle radiation attenuation, and high visceral fat were defined according to sex-specific previously published cut-offs [16, 17].
Statistical Analysis
The thresholds for CT image-derived body composition parameters to define sarcopenia and low muscle radiation attenuation have been determined for a population with mixed cancer disease locations [16, 18] whereas those for visceral obesity have been obtained from obesity-related research [19, 20]. The abovementioned thresholds for sarcopenia and low muscle radiation attenuation have already been used in pancreatic cancer patients [3, 4, 12, 13, 21, 22]. Besides this, candidate thresholds for sarcopenia based on SMA/body surface area (BSA) have been determined for gastric cancer patients [23]. However, there has been some criticism about the generalization of the reported thresholds due to ethnic and disease-site differences. As such, in recent studies with pancreatic cancer patients, different strategies to determine specific thresholds for each study population have been used, such as optimal stratification of total psoas area [5], sex-specific lowest quartile/tertile of SMA [9, 10], and receiver-operating characteristics (ROC) curve analysis [6, 11].
Since no thresholds for body composition have yet been established for the Portuguese population and bearing in mind that using thresholds not validated specifically for patients with pancreatic tumors may be misleading, we decided to use body composition variables in their continuous form, except for the Kaplan-Meier curves comparison, where dichotomization is necessary. From a statistical point of view, the use of continuous variables is a better option than discrete data, since continuous data convey more information. However, to account for gender-specific differences in body composition, variables were mean-centered in order to be scaled by sex. We decided not to use optimal stratification strategies, since this approach was considered unstable for our sample size.
Simple and multiple logistic regression were used to relate each variable with complications as Clavien-Dindo ≥IIIb. For continuous variables, linearity of the logit in the predictor was assessed using a cubic spline and the Wald test of linearity [24]. Only variables with a p value ≤0.25 or considered clinically relevant were selected for multiple logistic regression. Multicollinearity was also analyzed via the observation of variance inflation factors. A stepwise bothselection technique was used to create the multiple regression model. The ROC curve was computed and the respective area under the curve (AUC) was calculated to assess the accuracy of the model. The positive and negative predictive values (PPV and NPV) were also given. The association between major postoperative complications and type of surgery was assessed with the Fisher exact test.
Survival analysis was conducted with the Kaplan-Meier estimate, and survival curves were compared with the log-rank test. Body composition variables such as SMI and muscle radiation attenuation were dichotomized according to the lowest sex-specific quartile and VFA:SMA, with respect to the highest sex-specific quartile to allow for the comparison of survival curves.
First, 2 multiple Cox proportional hazards models with conventional clinical variables and body composition variables were adjusted and the C-statistic was computed to assess model prediction ability.
Lastly, a multiple Cox proportional hazards model was adjusted for 90-day survival, in order to allow the comparison between variables associated with 90-day survival and OS. In this comparison, we used the OS model containing both clinical and body composition variables which yielded the highest C-statistics. Data analysis was performed with SPSS v20 and R v3.0.2 and statistical significance was set at p ≤ 0.05.
Results
Population
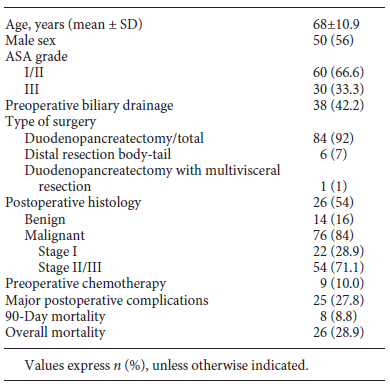
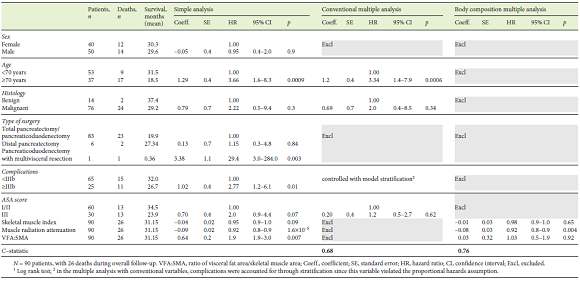
A total of 125 patients undergoing surgery for pancreatic tumors at our institution during the study period were screened for eligibility. Thirty-five patients were excluded because their diagnostic CT scan had been performed at another hospital. Excluded patients were compared to included patients, and no differences were found in demographics, postoperative complications, or mortality rate. Demographic and clinical data are presented in Table 1. Ninety patients were included, 56% of whom were male, and the mean age was 68 ± 10.9 years. Fourteen of 90 patients (16%) had evidence of benign or premalignant tumors on the surgical specimen (10 intraductal papillary mucinous neoplasms, 1 serous cystadenoma, 1 case of chronic focal pancreatitis, and 2 mucinous cysts). Among the patients with malignant tumors, although the large majority were pancreatic ductal adenocarcinomas, we also found 17 adenocarcinomas of the ampulla of Vater, 7 distal cholangiocarcinomas, 1 duodenal carcinoma, 3 malignant neuroendocrine tumors, and 1 acinar-cell carcinoma of the pancreas. Of the 90 patients, 92% had a total pancreatectomy or pancreaticoduodenectomy, 7% a distal pancreatectomy, and 1% a pancreaticoduodenectomy with multivisceral resection. The rate of major complications was 27.8%, the 90-day mortality rate was 8.8%, and 28.9% died during the study period. Survival was 81.1% at 1 year and 73.3% at 2 years. The mean follow-up period was 12.5 (0.26-49.8) months.
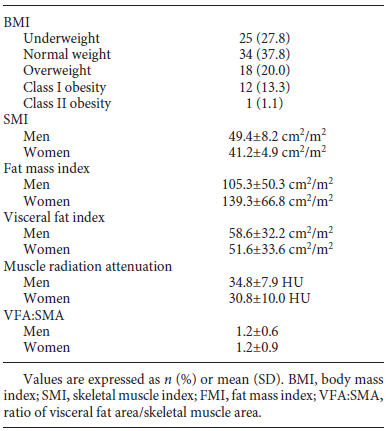
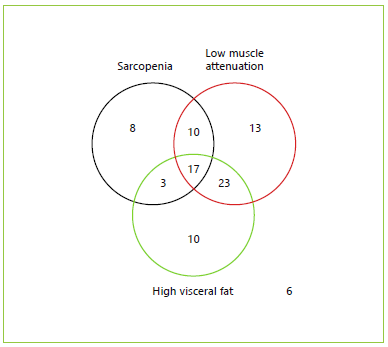
BMI categories and body composition parameters at diagnosis are presented in Table 2. The number of patients per body composition phenotype according to published cut-offs is shown in Figure 1. Mean BMI was 25.1 ± 4.03; 27.8% of the patients were underweight, 37.8% were of normal weight, 20.0% were overweight, 13.3% had class I obesity, and 1.1% had class II obesity. Furthermore, 2.2% patients presented sarcopenic obesity.
Postoperative Complications
Twenty-five of 90 patients had postoperative complications ≥IIIb. We observed that 24 of these 25 patients were referred for total pancreatectomy or pancreaticoduodenectomy, 1 for pancreaticoduodenectomy with multivisceral resection, and none for distal resection experienced major complications. In this analysis, we obtained a near-significant association between the type of surgery and major postoperative complications (p = 0.087). Table 3 shows simple and multiple logistic regressions exploring the association of each variable with the postoperative complications. Simple logistic regression showed that postoperative complications ≥IIIb were significantly associated with the visceral fat index and VFA:SMA, and an almost significant association was found for muscle radiation attenuation and ASA score. However, in the multiple logistic regression analysis, only VFA:SMA remained significantly associated with postoperative complications ≥IIIb. In this model, the odds of major complications were 2-fold greater per (an increase of 1) unit of VFA:SMA. Regarding ASA score, although it was perceived as relevant to major postoperative complications (since it was selected in the stepwise analysis), it lost statistical significance when adjusted for VFA:SMA. The AUC obtained through the ROC curve analysis was 0.691, which shows a fair discrimination ability of the selected model (Fig. 2). Sensitivity was 68.0%, specificity was 67.7%, PPV was 15.4%, and NPV was 55.3%. Lastly, we conducted a subset analysis including only patients with malignant disease, where VFA:SMA was the only variable to be selected for the final model with a near-significant association (OR 1.77, 95% CI 0.925-3.77, p = 0.10).
Table 3 Simple and multiple logistic regression with postoperative complications as the dependent variable
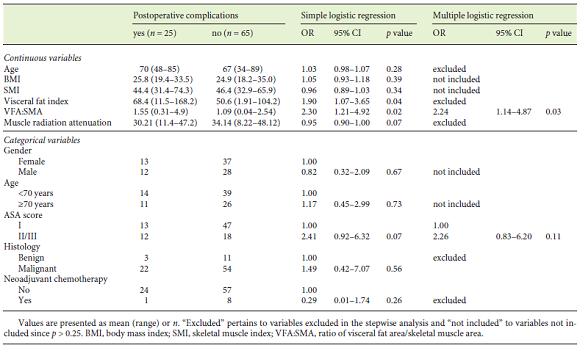
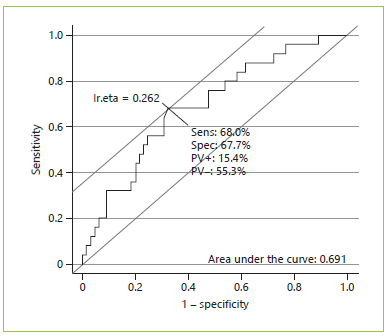
Fig. 2 Receiver-operating characteristics (ROC) curve for major complications as the dependent variable and ASA score and ratio of visceral fat area/skeletal muscle area as independent variables (n = 90). Sens, sensitivity; Spec, specificity; PV+, positive predictive value; PV-, negative predictive value.
Survival
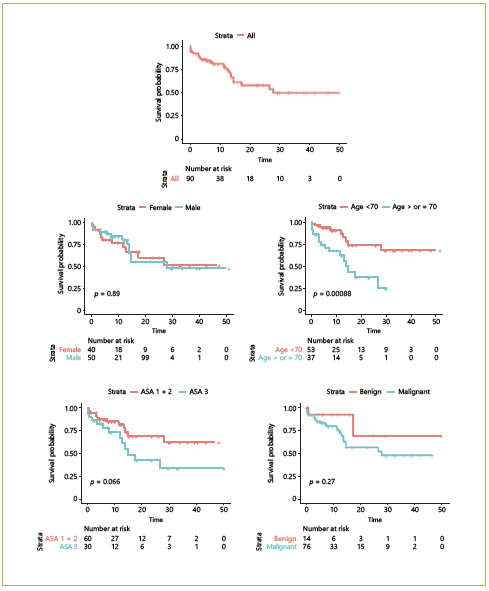
Estimated mean OS was 31.15 months. Kaplan-Meier survival curves for OS as well as their comparison with clinical and body composition variables are reported Figures 3, 4, 5. Dichotomization of body composition parameters was conducted using the first sex-specific quartile for SMI (males 43.9 cm2/m2 and females 37.2 cm2/ m2), muscle radiation attenuation (males 30.9 HU and females 23.42 HU), and the third sex-specific quartile for the VFA:SMA (males 1.52 and females 1.67) to allow for the Kaplan-Meier curves comparison. Comparison of survival curves was also conducted for BMI categories (low/normal weight vs. overweight/obese), but no statistically significant differences were found (p = 0.332).
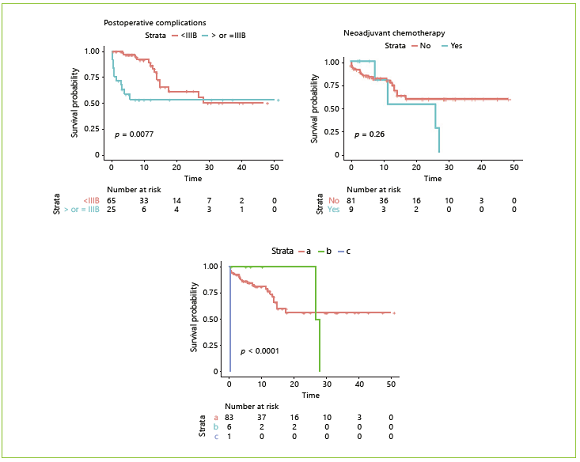
Fig. 4 Overall survival according to clinical variables: sex, age, ASA score, disease malignancy, neoadjuvant chemotherapy, postoperative complications, and type of surgery. a Total pancreatectomy or pancreaticoduodenectomy. b Distal pancreatectomy. c Pancreaticoduodenectomy with multivisceral resection (n = 90). IIIB, Clavien-Dindo class IIIb.
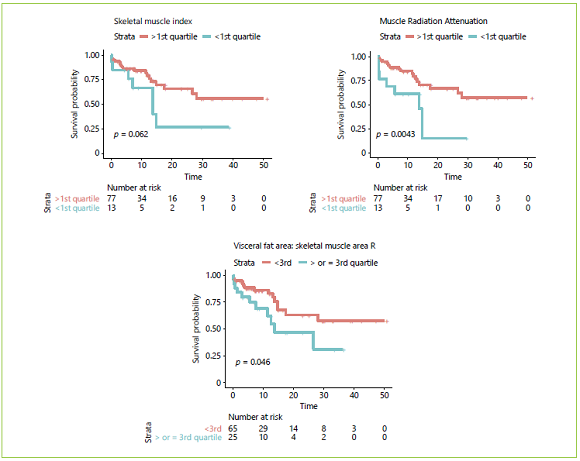
Fig. 5 Overall survival according to body composition variables dichotomized according to quartiles: skeletal muscle index, muscle radiation attenuation, and ratio of visceral fat area/skeletal muscle area (VFA:SMA) (n = 90).
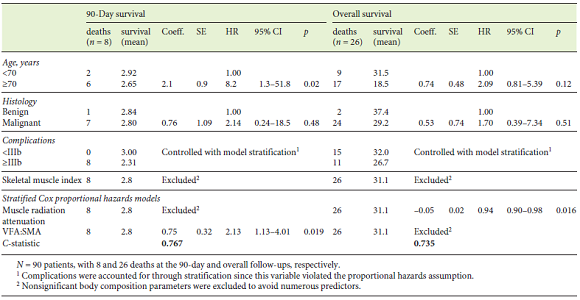
Table 4 presents the results of the simple and multiple analyses for OS. Regarding clinical variables, in the simple analysis, shorter survival was observed in patients aged ≥70 years, those submitted to pancreaticoduodenectomy with multivisceral resection, with postoperative complications ≥IIIb, and when a near-significant p value was found for ASA score (I/II vs. III). Regarding body composition variables, an increase of 1 unit in muscle radiation attenuation was associated with an 8% reduction in the estimated risk of death, whereas an increase of 1 unit in VFA:SMA was associated with an increase of 90% in the estimated risk of death. Lastly, a near-significant association was found between OS and SMI, where an increase of 1 unit was associated with a 5% reduction in the estimated risk of death.
Two multiple proportional hazards Cox models were adjusted using variables significantly associated with OS or considered clinically pertinent, and the C-statistics were compared. In the first model, only conventionally used clinical variables were included, namely age, ASA score, histology, and postoperative complications. In this model, both age and postoperative complications were significantly associated with OS. The estimated risk of death was 3.34 times greater in patients aged ≥70 years. HRs for major complications could not be computed because this variable was adjusted by stratification as it violated the proportional hazards assumption. At first, we decided not to include the type of surgery because most patients were submitted to total pancreatectomy or pancreaticoduodenectomy, and there were very few patients who underwent distal pancreatectomy and multivisceral resection which can influence estimates. However, bearing in mind the clinical relevance of this variable, we performed the same analysis including the type of surgery; this did not alter the previous results or increase the performance of the model (the C-statistics remained the same), so we decided to not include it after all to avoid overfitting (data not shown). A second model was adjusted using body composition variables. In this model, we included SMI, muscle radiation attenuation, and VFA: SMA. The only significant variable was muscle radiation attenuation, where an increase of 1 HU was associated with an 8% reduction in estimated risk of death. Interestingly, the model which included 3 body composition variables had a discrimination ability (a C-statistic of 0.76) superior to the model which included 4 conventional clinical variables (a C-statistic of 0.68).
To compare postoperative survival with OS, Table 5 shows the results obtained for the model that yielded the highest C-statistics with regard to OS and the results of the Cox proportional hazards model for the analysis of 90-day survival. Ninety-day survival was associated with age, with patients aged ≥70 years displaying an 8.2-fold greater estimated risk of postoperative death compared with patients aged < 70 years. Although in this analysis we could not compute the HR for postoperative complications ≥IIIb due to model stratification, it is worth noting that all patients who had died at 90 days had experienced major complications. Also, the estimated risk of death was 2 times higher per (an increase of 1) unit of VFA:SMA adjusted for age, histology, and postoperative complications.
On the other hand, when we analyzed OS, age was no longer a determinant factor. With regard to body composition parameters, the results differed from those for 90-day survival, and muscle radiation attenuation was the only significant variable where an increase in 1 unit was associated with a 6% reduction in estimated risk of death, independently of age, histology, and postoperative complications.
Finally, the 90-day survival and OS analyses were conducted only for patients with malignant disease, and similar results were obtained. In a model that adjusted for postoperative complications and age, VFA:SMA (HR 2.02, 95% CI 1.07-3.81, p = 0.03) was significantly associated with 90-day survival. In a model adjusted for postoperative complications and age, muscle attenuation was significantly associated with OS (HR 0.94, 95% CI 0.89-0.99, p = 0.019).
Discussion
In this observational study performed in a single reference center for pancreatic surgery, we observed that body composition is a major determinant of the outcome of surgically treated patients. We found that, for predicting survival, the discrimination achieved with a model that includes 3 body composition parameters proved superior to a model with 4 conventional clinical variables such as age, ASA score, disease histology, and postoperative complications. With regard to postoperative complications, a major determinant of OS, we observed a significant association with VFA:SMA independently of ASA score. It is worth pointing out that, in our sample, only 2 patients met conventional criteria for sarcopenic obesity.
Considering the high morbidity and mortality associated with pancreatic surgery, our findings are highly relevant. Although age, ASA score, and disease stage are non-modifiable factors, we can certainly aim at modifying preoperative body composition, especially during neoadjuvant chemotherapy. This is being more frequently prescribed even in tumors which seem resectable upfront. Criteria for considering a tumor as borderline resectable have been recently expanded and, as of today, neoadjuvant chemo/chemoradiotherapy is now strongly recommended in a substantial proportion of operated patients, if not all [25-28]. This period usually lasts 6 months or even more, which is an excellent opportunity to intervene with combined programs of exercise and/or dietary intervention aimed at modifying body composition.
Most studies that have addressed the relationship between body composition and the clinical outcome of pancreatic surgery patients focused mainly on skeletal muscle tissue and OS, with contradictory results. The studies have approached this issue using different methods to tackle skeletal muscle tissue, namely L3 CT scan-derived sarcopenia [4, 10, 16, 29], SMA loss [30], accelerated loss of muscle mass [20], total psoas area [1, 11] and volume [5], which have been associated with shorter OS in pancreatic cancer patients submitted to surgery or palliative care.
In contrast, other studies failed to demonstrate such an association [9, 22], or were only able to show an association with sarcopenia if BMI was accounted for (which may be thought of as a proxy of body fatness) [8, 12]. Some studies included patients submitted to curative and palliative procedures which obviously have different outcomes [13]. In this study, we enrolled patients submitted to curative surgery only; 84% had malignant tumors while the remaining ones had premalignant lesions, and all patients were treated by the same team of 3 surgeons. Although skeletal muscle per se was not associated with a worse prognosis, it is worth noting that both VFA:SMA and muscle attenuation, which incorporate both fat and skeletal muscle tissue, were strong determinants of postoperative complications, 90-day survival, and OS, respectively. This suggests that these body compartments should not be considered isolated as they seem to exert a joint influence on the final outcome.
Notably, most studies published so far have addressed OS; only a small number have investigated the effect of body composition on postoperative survival. Joglekar et al. [31] found that sarcopenia was an independent predictor of grade-III complications, but they did not analyze visceral fat or muscle attenuation. In our study series, VFA:SMA was independently associated with 90-day survival and postoperative complications on multivariate analysis, while ASA score lost significance. Our findings are in line with the only published study to investigate the influence of VFA:SMA on 60-day mortality, and found that a VFA:SMA exceeding 3.2 and ASA III were the strongest predictors of mortality [3].
In our study, muscle radiation attenuation was found to be the most relevant body composition parameter associated with OS independently of age, postoperative complications, and disease histology. This result supports the hypothesis that muscle quality may be one of the most important body composition parameters influencing OS. In agreement with our results, van Dijk et al. [13] also found that low skeletal-muscle radiation attenuation was associated with worse OS and a high skeletal/visceral adipose tissue index was related to an increased surgical-site infection rate. In this particular study, 50% of patients had non-pancreatic cancer and 30% of them were submitted to palliative procedures which substantially increased the heterogeneity of the study population. In a recent study, Stretch et al. [9] found that myosteatosis was associated with an increase in postoperative complications but found no correlation between body composition and OS. Of note, the reported incidence of major postoperative complications and deaths in their study was exceedingly low, which could explain this lack of correlation. They concluded that sarcopenia and myosteatosis represent 2 separate and distinct clinical phenotypes, but they did not include VFA:SMA in their analysis.
To our knowledge, this is the first study examining the effect of body composition in its most holistic perspective including the ratios between visceral fat and skeletal muscle tissues in both postoperative outcomes and OS. Although well-powered prospective studies are still needed, our results suggest that muscle radiation attenuation may be an independent prognostic factor of OS and that VFA:SMA is significantly associated with 90-day survival and postoperative complications.