Introduction
Pancreatic and peripancreatic collections (PPC) are local complications of an acute pancreatic event (acute pancreatitis, pancreatic trauma or pancreatic surgery) or a chronic pancreatic injury (chronic pancreatitis or autoimmune pancreatitis) [1, 2]. PPC can occur secondary to pancreatic/peripancreatic inflammation or to a disruption of the main pancreatic duct and/or its side branches [1]. PPC are a known local complication of acute pancreatitis and according to the revised Atlanta classification [3] are categorized into four types: (1) acute peripancreatic fluid collection, (2) pseudocyst, (3) acute necrotic collection and (4) walled-off necrosis (WON) (Table 1).
Most acute peripancreatic fluid collections remain sterile and resolve spontaneously without intervention, so that drainage should not be performed because of the risk of infecting an otherwise sterile collection [3, 4]. Pseudocysts may develop in 5-15% of patients with acute pancreatitis [5], the majority of which are asymptomatic and present spontaneous regression or resolution (7-60%) [6]. Pseudocysts may also complicate 20-40% of cases of chronic pancreatitis, and they are usually asymptomatic as well [7]. For acute necrotic collections, a prospective observational study showed that 43% presented spontaneous remission and 51% matured into WON. Furthermore, most WON cases also resolved spontaneously or were persistent but asymptomatic, with only 21% of patients requiring an intervention [8]. Therefore, intervention is needed for a minority of patients.
In this review article, the Portuguese Group for Ultrasound in Gastroenterology (GRUPUGE) presents a perspective on the potential role of endoscopic ultrasound (EUS)-guided drainage of peripancreatic collections, addressing the selection criteria and technical issues of different techniques and analysing emerging data on their efficacy and safety.
Methods
A systematic literature search and review was performed until January 2020, using PubMed, MEDLINE, Scopus and Google, using the keywords “acute pancreatitis,” “pancreatic pseudocyst,” “walled-off pancreatic necrosis,” “transmural drainage of pancreatic fluid collections,” “endoscopic ultrasound-guided drainage of peripancreatic collections” and “endoscopic necrosectomy.” Prospective/comparative studies and international consensus statements/management guidelines were preferred. The final manuscript was revised and approved by all the members of the Governing Board of the GRUPUGE.
Indications for Drainage
It is currently recommended to perform drainage of a PPC, usually a pseudocyst or a WON, in the following cases:
− Proven or presumed infection of the collection [1, 4, 9, 10], which occurs more frequently in necrotic collections and is associated with increased mortality (12-39%) [11]; clinically manifested as new decompensation in a previously stable patient, persisting or increasing elevation of inflammatory parameters (fever, leucocytosis and C-reactive protein), or new or prolonged organ failure or increased need for cardiovascular, respiratory or renal support despite optimal medical therapy in the absence of an alternative source of infection; demonstrated by imaging findings of gas within the collection
− Significant symptoms related to the collection [1, 9-11], such as persistent abdominal pain (often exacerbated by eating), gastric outlet obstruction (nausea and vomiting), obstructive jaundice (due to biliary compression) or pancreatic leakage due to a disrupted duct manifested as pancreatic ascites or pleural effusion
− Abdominal compartment syndrome [9]
The size of the PPC by itself is no longer considered a reason for intervening, as the majority of PPC tend to resolve spontaneously over time [11]. In case of an indication for intervention, in particular for endoscopic drainage, it is important to differentiate a pseudocyst from a WON. As pseudocysts are fluid-only collections, drainage is usually sufficient [6]. However, WON contains fluid and solid necrotic debris and may need further debridement of necrotic tissue after initial drainage [6]. For this reason, endoscopic treatment of WON has a lower success rate and higher complication rate and leads to more frequent reinterventions and longer hospital stays compared to drainage of pseudocysts [12]. Magnetic resonance imaging and EUS perform better than computed tomography (CT) for evaluation of the presence and extent of solid necrotic debris within a collection [13].
Role of EUS in PPC Drainage
Over the last decade, management of PPC has evolved significantly, shifting from primary open surgery towards minimally invasive techniques. In the trials by the Dutch Pancreatitis Study Group [14, 15], EUS-guided drainage and endoscopic necrosectomy were pointed out as less invasive alternatives to surgery for infected necrotizing pancreatitis; no difference in mortality and major morbidity was found between groups, while the rate of pancreatic fistulas and the length of hospital stay were lower in the endoscopy group [14].
Currently, EUS-guided transluminal drainage should be the first-line treatment option [9, 10]. It is a minimally invasive procedure that involves the creation of a fistulous tract between the PPC and the gastric or duodenal lumen (cystogastrostomy or cystoduodenostomy), followed by placement of a stent to keep the fistula patent and allowing transluminal PPC drainage [6]. It allows management of collections that do not bulge into the gastric lumen and assesses in real time the wall maturity and collection content (fluid only or with the presence of solid necrotic debris), guiding proper treatment strategies. The procedure should be delayed, whenever possible, until at least 4 weeks after initial presentation to allow wall maturation and better necrotic tissue demarcation and liquefaction [9]. As previously mentioned, EUS-guided transluminal drainage is usually sufficient for pseudocysts. However, for WON, endoscopic necrosectomy may be required after drainage.
Other approaches available for PPC management include conventional blind endoscopic transluminal drainage, transpapillary drainage, percutaneous drainage (under ultrasonography or CT guidance) and surgical drainage (laparoscopic necrosectomy via the transperitoneal approach, video-assisted retroperitoneal debridement or open surgical necrosectomy). They can also be combined in complex cases, for example, dual endoscopic and percutaneous drainage, a surgical step-up approach (percutaneous catheter drainage followed by video-assisted retroperitoneal debridement), or endoscopic and surgical drainage [9].
EUS-guided transmural drainage has had similar technical and clinical success to percutaneous or surgical approaches, but with lower morbidity and costs and better quality of life [6, 14, 16, 17]. It has also had greater technical success than conventional blind endoscopy [18, 19].
Given the several treatment modalities available, patients with PPC should ideally be managed by a multidisciplinary team, considering local expertise, patient comorbidities and type of collection.
Pre-Drainage Evaluation
Before endoscopic drainage, an evaluation of the patient and the PPC should be performed [1, 6]:
− History and physical examination of the patient, including a documented history of acute or chronic pancreatitis, pancreatic surgery or trauma that may explain the collection
− Laboratory evaluation with coagulation profile and management of antithrombotic/anticoagulant agents as appropriate for high-risk procedure for bleeding
− CT scan assessing the relation between the collection and the stomach or duodenum, giving information about anatomic details (vascular structures, signs of portal hypertension, arterial pseudoaneurysms, ascites, large or atypically located gallbladder, multiple collections) and, although less accurate, possibly also suggesting the presence of solid necrotic debris within the collection
− EUS imaging (possibly at the same session as EUSguided drainage) to localize the collection and its contact zone with the gastric/duodenal lumen, to confirm the development of a mature wall around the collection, to evaluate the presence and extent of solid necrotic debris and to assess for interposed vessels by Doppler mode; furthermore, EUS in combination with fine-needle aspiration and contrast-enhanced ultrasound is helpful in distinguishing a PPC from a cystic neoplasm, avoiding misdiagnosis and guiding proper management decisions
Regarding antibiotic therapy, patients undergoing EUS-guided drainage for infected PPC should continue the previously instituted treatment [1]. The role of prophylactic antibiotics in patients undergoing EUS-guided transmural drainage for non-infectious reasons has not been studied [20]. Nevertheless, patients usually receive periprocedural antibiotics followed by a short course thereafter (often for 3-5 days) [1, 20].
EUS-guided drainage should also be performed under deep sedation or anaesthesia given its complexity, and with the availability of surgical, intensive care and interventional radiology support in the event of complications [10, 21].
EUS-Guided Transmural Drainage: The Procedure
Using the Standard Procedure (Fig. 1)
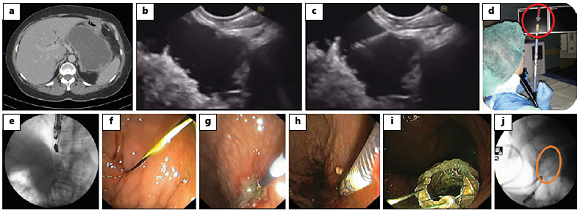
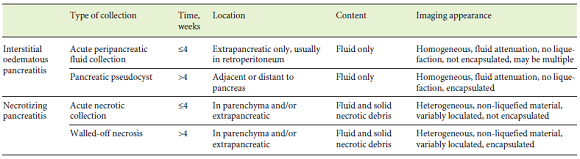
Fig. 1: EUS-guided transmural drainage using a standard approach. a, b Computed tomography (a) and EUS (b) images obtained 6 weeks after a severe pancreatitis episode showing a large homogeneous fluid collection between the pancreatic corpus and the stomach, with a well-defined wall; the findings were consistent with a pseudocyst. c Via a transgastric approach, puncture of the collection using 19-G fine-needle aspiration was performed. d Aspiration of purulent fluid from the collection. e, f A 0.035-inch guidewire is introduced through the needle (e) and is coiled within the pseudocyst under fluoroscopic guidance (f). g The fistulous tract is dilated using a 10-Fr cystostome. h Placement of a lumenapposing metal stent (BCF covered Diabolo shape, Hanarostent®). i Proximal flange of the stent deployed in the stomach. j Stent deployment confirmed by fluoroscopy. EUS, endoscopic ultrasound.
The standard procedure should be performed in a room with fluoroscopic imaging, as both ultrasonography and fluoroscopy guidance are needed for stent deployment. A therapeutic linear echoendoscope with a working channel of 3.7-3.8 mm is required in order to enable the insertion of stents or a nasocystic catheter.
1. Begin the procedure with location of the collection and its contact zone with the gastric/duodenal lumen; the distance between the collection and the gastroduodenal wall should not exceed 10 mm in order to smooth the procedure and avoid adverse events [22]
2. Assess for interposed vessels by Doppler mode and determine the optimal site for puncture
3. Puncture the collection using 19-G fine-needle aspiration (a sample may be aspirated and sent for laboratory analysis, such as microbiology); a 0.035-inch guidewire is introduced through the needle and is coiled within the pseudocyst under fluoroscopic guidance
4. Dilate the tract sequentially using endoscopic retrograde cholangiopancreatography cannulas, Soehendra biliary dilators or 6- to 15-mm over-the-wire biliary balloons; alternatively, a 10-Fr cystostome or a needle knife catheter may be used for dilation
5. For double pigtail plastic stent (PS) placement, introduce an additional guidewire through the fistulous tract and place the two PS, usually of a calibre of 7-10 Fr
6. For fully covered self-expandable metallic stent or lumen-apposing metal stent (LAMS) placement, introduce the stent delivery device through the tract over the wire and deploy the stent
Using a Cautery-Enhanced LAMS (Fig. 2)
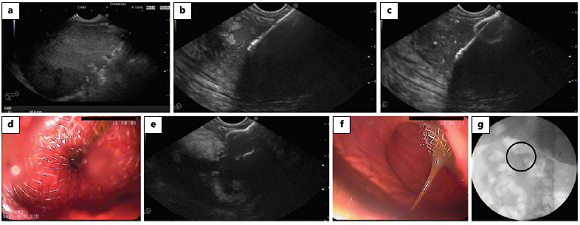
Fig. 2: EUS-guided transmural drainage using a cautery-enhanced lumen-apposing metal stent (Hot AXIOS). a EUS image obtained 4 weeks after severe pancreatitis showing a heterogeneous fluid collection with solid debris and a well-defined wall, localized between the pancreatic corpus and the posterior wall of the stomach; the findings are consistent with walled-off necrosis. b Via the transgastric approach and under EUS guidance, the catheter is advanced into the collection with an electrocautery tip. c The distal flange of the stent is deployed inside the collection. d The proximal flange of the stent is released and visible in the stomach. e Removal of the delivery system, with the stent deployed across the gastric wall into the collection. f Proximal flange of the stent deployed in the stomach. g Stent placement confirmed by fluoroscopy. EUS, endoscopic ultrasound.
The delivery system comes with an electrocautery wire at the distal tip of the catheter designed for simultaneous puncture and tract dilation followed by stent deployment, without the need for guidewire placement. After choosing the optimal site for puncture, the electrocautery tip allows passage of the deployment device into the PPC followed by stent placement. With this delivery system, the procedure is performed under EUS and/or endoscopic guidance, whereas fluoroscopic control is not needed.
Stents in Transmural Drainage
Currently available stents include PS, fully covered self-expanding metal stents (FCSEMS) and LAMS.
Using PS (Fig. 3)
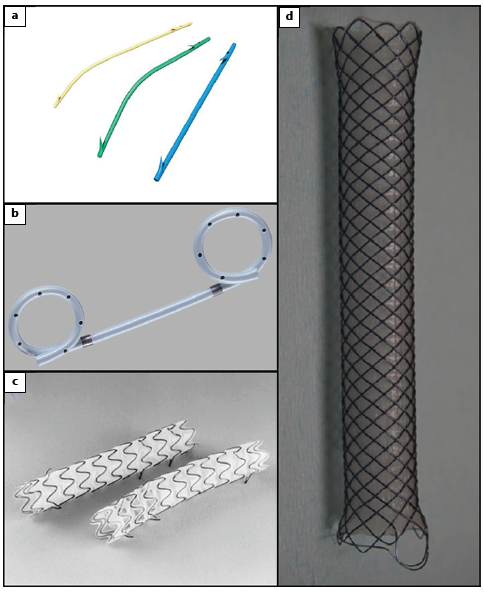
Fig. 3: Plastic and fully covered self-expanding metal stents. a Straight plastic stents. b Double pigtail plastic stent. c VIABIL biliary metal stents (GORE®). d Wall- Flex biliary metal stent (Boston Scientific ®).
Traditionally, PS were used for transmural drainage [6]. The type of PS used may be straight or pigtail. Double pigtail PS are more frequently chosen, since they are less prone to migrate into or out of the collection [1]. Moreover, straight stents can cause delayed bleeding from friction of the stent against the wall of the cavity or stomach as the collection resolves [1].
For pancreatic pseudocysts, transmural stenting using double pigtail PS showed high efficacy (85.1-90.8%) [23-25]. Nevertheless, treatment success is significantly lower for drainage of WON (69.5-81.8%) [23-25], which may be explained by the small diameter of PS and the presence of solid debris in WON that causes stent occlusion and hampers drainage through the fistulous tract [11]. Therefore, the main disadvantage of PS is their small bore with need to access the collection cavity several times or to use various guidewires to place multiple stents in order to maintain drainage, which is labourintensive and time-consuming [26]. Furthermore, a 10-Fr PS can be hard to advance and deploy through the 3.7- to 3.8-mm channel of the echoendoscope [26], so narrower stents are frequently used (7 or 8.5 Fr). Repeat balloon dilation of the fistulous tract is also needed to permit endoscopic necrosectomy.
Using FCSEMS (Fig. 3)
Straight biliary or oesophageal FCSEMS have been tried in patients with PPC due to theoretical advantages over PS. Given their larger stent diameter, they can facilitate drainage of both liquid and the viscous necrotic debris, decreasing the risk of stent occlusion and the need for repeat procedures [2, 25]. They also may shorten the duration of the procedure, since they require a single access to the collection for stent delivery, rather than multiple access points as required for the deployment of multiple PS [2]. Due to its tubular configuration lacking anchoring flanges, the main disadvantage of FCSEMS is stent migration [26], so that a single double pigtail PS is usually placed within the FCSEMS in order to maintain stent patency as the cavity resolves as well as to prevent migration [1].
A few studies reported great treatment success with EUS-guided drainage of pseudocysts using FCSEMS (78-100%) [2]. In a retrospective cohort study evaluating EUS-guided drainage of pancreatic pseudocysts [27], FCSEMS achieved higher complete resolution rates (98 vs. 89%; p = 0.01) and fewer early adverse events (14 vs. 31%, p = 0.008) than PS.
Using LAMS (Fig. 4)
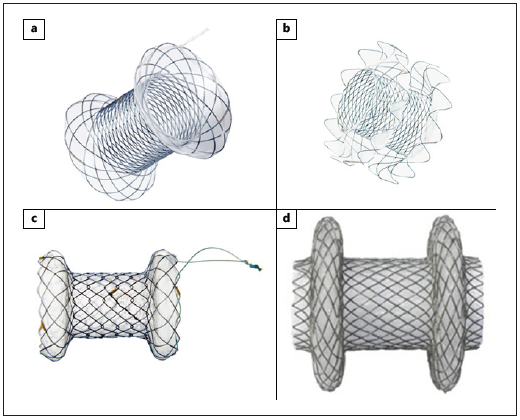
Fig. 4: Lumen-apposing metal stents. a NAGI stent (TaeWoong Medical). b SPAXUS stent (TaeWoong Medical). c BCF covered Diabolo shape stent (Hanarostent ®). d Hot AXIOS stent (Boston Scientific).
LAMS have been increasingly used in PPC management, especially for WON, because the larger diameter of the stent facilitates drainage and can accommodate the repeated endoscope entry and exit necessary for necrosectomy [1]. LAMS also have a “dumb-bell” configuration with two wide flanges that provides stable apposition between the digestive wall and the collection wall, avoiding stent migration [1].
Some authors suggest placing a single double pigtail PS within the LAMS in order to allow egress of fluid in the event that necrotic material impacts the LAMS lumen, to protect the inner wall of the collection and prevent contact-related delayed bleeding, allow retrieval of buried LAMS (if it occurs) and create space if long-term double pigtail stents are needed to manage disconnected pancreatic duct syndrome [1].
In a meta-analysis [28], LAMS had high technical and clinical success in the management of both pancreatic pseudocysts (86-99% and 93-99%, respectively) and WON (82-100% and 64-98%, respectively) [28].
Plastic versus Metal Stents
In an RCT comparing EUS-guided drainage for symptomatic WON using LAMS or double pigtail PS [29, 30], there was no significant difference in total number of procedures performed, treatment success, readmissions and length of hospital stay. The procedure duration was shorter with LAMS (15 vs. 40 min; p < 0.001), and although the procedure costs were higher with LAMS, overall treatment costs were similar between groups. An interim audit was performed due to a higher-than-anticipated procedural adverse event rate in the LAMS cohort (50 vs. 0%; p = 0.019) observed 3 or more weeks after LAMS placement. The authors hypothesized that the wide diameter of the LAMS facilitated better drainage of the necrotic contents, leading to faster WON resolution. Due to its immobility, the LAMS remained in place, causing friction with the adjacent vasculature surrounding the necrotic cavity, thereby precipitating bleeding, occlusion and buried stent syndrome. Therefore, an amendment was made to the study protocol, including a CT scan 3 weeks after LAMS placement, followed by stent removal if WON had resolved. After protocol amendment, there was no significant difference in adverse events between cohorts.
A prospective case control study [31] was also conducted to compare biliary FCSEMS and LAMS in patients with PPC drained under EUS guidance. The use of a double pigtail PS to prevent metal stent migration, as well as the use of a nasocystic catheter for cavity lavage, was significantly less frequent with LAMS than with FCSEMS (33 vs. 100% and 13 vs. 58%, respectively; p < 0.0001). All procedures for LAMS placement took less than 30 min, while all FCSEMS placements took over 30 min (p = 0.0001). The LAMS cohort also had greater clinical success, but not statistically significantly so (96 vs. 82%, p = 0.055), as well as a lower adverse event rate (4 vs. 18%; p = 0.04).
In a systematic review including 5 retrospective cohort studies comparing PS and LAMS [32], overall WON resolution was more likely to occur with LAMS than with PS (91.5 vs. 80.9%; OR = 2.5; 95% CI: 1.4-4.3; p = 0.001). Furthermore, 2 systematic reviews, including comparative studies between plastic and metal stents (FCSEMS and/or LAMS) [23, 25], also demonstrated a higher rate of clinical success and a lower rate of adverse events with metal stents for both pseudocysts and WON. In contrast, 2 other systematic reviews [24, 33] showed no difference in overall treatment success or rates of adverse events between PS and metal stents.
Based on current evidence, the European Society of Gastrointestinal Endoscopy (ESGE) suggests either PS or LAMS for initial endoscopic transmural drainage [9]. Pseudocysts will most likely resolve with transmural placement of two double pigtail PS or one metal stent (FCSEMS or LAMS). For management of WON, some experts suggest the use of metal stents (preferentially LAMS) as first-line treatment given the apparent higher efficacy in drainage and the likelihood of endoscopic necrosectomy. However, more data are needed from well-designed prospective trials in order to clarify the effective superiority of metal stents (and LAMS in particular).
Stent Removal after Drainage
The PPC recurrence rate in the first year after stent removal is reported to be around 10-38% [11]. Prolonged transluminal stenting with PS may maintain the patency of the fistulous tract and prevent recurrence. However, retrieval of LAMS is recommended within 4 weeks after placement to prevent stent-related adverse effects [9].
Prior to stent removal, imaging of the main pancreatic duct by CT, magnetic resonance cholangiopancreatography (preferably with secretin) or endoscopic retrograde cholangiopancreatography is suggested in order to rule out disruption and disconnected pancreatic duct syndrome [9, 13]. Disruption of the pancreatic duct may occur in 40-60% of patients with PPC and leaves a functioning body or tail disconnected from the head of the pancreas, with ongoing leakage of pancreatic exocrine secretions leading to persistence or recurrence of the PPC [11]. Patients with partial rupture of the pancreatic duct benefit from placement of a transpapillary pancreatic stent to bridge the site of the leak and facilitate preferential drainage via the main pancreatic duct (since transmural drainage does not directly treat the pancreatic duct disruption) [34]. When the disruption is complete or stent bridging of a partial rupture fails, long-term indwelling of transmural PS is recommended after PPC drainage [9]. In case a metal stent (FCSEMS or LAMS) was placed for transmural PPC drainage, this should be replaced by a PS [9].
Endoscopic Management of Necrotic Debris
As previously mentioned, treatment success for WON is significantly lower than for pseudocysts [12], because clearing of solid necrotic debris may be incomplete with only drainage, and further management is needed.
Several approaches have been proposed for endoscopic management of necrotic debris: (1) irrigation, (2) the multiple transluminal gateway technique, (3) endoscopic necrosectomy and (4) the dual-modality drainage technique.
Irrigation Technique
In order to facilitate debridement of necrotic tissue, a nasocystic catheter (5-7 Fr) may be inserted during the access procedure to the PPC, alongside the PS or within the deployed metal stent [9]. Through the nasocystic tube, continuous or sequential irrigation of the PPC with normal saline solution is applied during the first 48-72h or between each necrosectomy session (for a daily volume of 500-1,500 mL). In a retrospective study using double pigtail PS [35], the placement of a nasocystic tube led to a significantly greater treatment success at the 1-month follow-up (85 vs. 63%; p = 0.03), but no significant differences at 12 months (79 vs. 58%; p = 0.059), compared to PS alone. A retrospective study using LAMS [36] also showed no statistically significant difference in the overall success of WON resolution with and without nasocystic tube placement at the 3-month follow-up (90.9 vs 95.6%; p = 0.59). In 1 study, antibiotics were also added to the irrigation solution according to the microbiological findings [37]. Therefore, it is unclear if this technique has any advantage, especially when combined with a metal stent (LAMS in particular).
Another irrigation technique is to perform lavage of the collection cavity through the working channel of the endoscope during the necrosectomy session [9]. In 1 noncomparative study including 12 patients with WON [38], lavage sessions were performed by flushing normal saline solution (500-1,500 mL) through the LAMS using a water jet system, followed by total aspiration of the flushed solution and removal of all non-adherent necrotic material. The authors reported a clinical success in all patients, without the need for direct mechanical necrosectomy [38]. Some studies also employed lavage with an antibacterial solution [39, 40] or hydrogen peroxide-saline solution [30] in order to remove bacterial biofilm and assist with debridement.
Multiple Transluminal Gateway Technique
This modality consists of the creation of 2-3 fistulous tracts (rather than a single one) between the WON and the gastric or duodenal lumen in order to facilitate better drainage of necrotic material and fluid [41]. One tract may serve for irrigation through a nasocystic tube and the others for drainage through deployed stents [6, 41]. The multiple transluminal gateway technique can be considered for patients with multiple or large (> 12-cm) WON, with a suboptimal response to single transluminal gateway drainage, or when the position of first access hampers the introduction of the endoscope into the cavity for necrosectomy [9]. Two retrospective studies [42, 43] showed greater treatment success with the multiple transluminal gateway technique than with single drainage using PS. A case of successful use of LAMS in multiple transluminal drainage has also been published [44].
Endoscopic Necrosectomy
Another technique for the management of necrotic tissue consists of its mechanical removal from the collection cavity; it is named endoscopic necrosectomy. It can be performed by removal of necrotic debris using accessories or devices introduced from the digestive lumen into the collection (transluminal endoscopic necrosectomy) or by insertion of the endoscope into the cavity to remove the necrotic debris (direct endoscopic necrosectomy) [9] (Fig. 5).
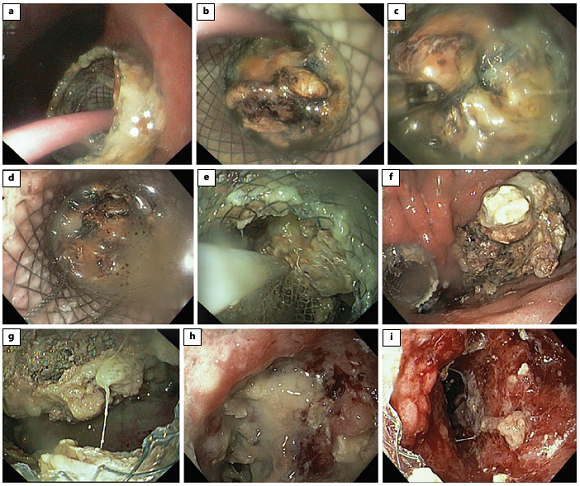
Fig. 5: Direct endoscopic necrosectomy. A nasocystic catheter, placed through the stent (lumen-apposing metal stent) inside the cavity, is removed (a, b). Using forceps (c), a polypectomy snare (d) and a net (e), necrotic debris is removed to the stomach (f). Progressive resolution of the walled-off necrosis is seen with necrosectomy (g, h), showing pink granulation tissue on the cavity wall (i).
The location of drainage is important if necrosectomy is foreseen, because a too proximal (fundus, cardia) or a too distal (antrum) access may compromise the direct introduction of the endoscope into the cavity and render its manipulation more difficult [9]. Concerning stent choice, as previously mentioned, LAMS carry some theoretical advantages over other stents: (1) a larger diameter enabling passage of an endoscope through the stent for direct endoscopic necrosectomy, (2) avoidance of multiple stents or repeated balloon dilations and (3) wider flanges that provide stable apposition [1, 11]. However, prospective studies are needed to confirm the effective superiority of LAMS.
There is no consensus regarding the optimal timing for direct endoscopic necrosectomy. It is typically delayed a few days after stent placement, in order to allow maturation of the fistulous tract and decrease the risk of dislodgement, and also because some WON can resolve with stent drainage alone [45]. Nevertheless, a retrospective multicentre study [46] compared immediate (same session as stent placement) and delayed (1 week after stent placement) direct necrosectomy using LAMS and showed no significant difference in clinical success rates between the two groups. Overall adverse events were similar, though stent dislodgements were more frequent in the immediate-necrosectomy group (4.3 vs. 0%; p = 0.016) and all occurred at the index endoscopy during necrosectomy; the stent was successfully repositioned with grasping forceps in all patients without complications.
Data comparing the types of endoscope used for endoscopic necrosectomy are lacking. The ESGE suggests the use of a therapeutic gastroscope due to its larger working channel that may facilitate evacuation of fluids and entry of equipment to be used for necrosectomy [9].
No specifically designed accessories or devices are available for necrosectomy. It is performed by a combination of irrigation, sucking debris through the working channel and removing necrotic material with a removal device (such as polypectomy snares, nets, tripod retrieval forceps, grasping/rat-tooth/pelican forceps, Dormia or other stone removal baskets, or EndoRotor devices) [9, 47]. There are no studies comparing the efficacy or safety of these accessories. Snares and baskets might be preferred for the primary attempt as they are safe and quite effective [9]. The EndoRotor (Interscope Medical, Whitinsville, MA, USA), a novel automated non-thermal mechanical system designed for polyp resection in the gastrointestinal tract, is also being studied in the setting of necrosectomy and allows the necrotic tissue to be sucked into a catheter using negative pressure, cut by a rotating blade and removed to a vacuum container [47]. The ESGE suggests restraint regarding the use of high-flow water jet systems, hydrogen peroxide or vacuum-assisted closure systems [9].
Concerning insufflation during transmural drainage and/or necrosectomy, the utilization of carbon dioxide instead of room air is recommended in order to reduce the risk of air embolism [9, 48]. Suspected or likely air embolism was reported in 0.9-2% of the procedures when air insufflation was used during necrosectomy [9]. Carbon dioxide is rapidly absorbed and highly soluble in water or blood, and no cases of air embolism have been documented with carbon dioxide insufflation [9]. Nevertheless, gas insufflation should be minimized during necrosectomy to maintain minimal gas pressure within the retroperitoneum [9].
Again, no criteria for WON resolution have been defined, but some have been proposed: more than 40% necrotic tissue disappearance within 1 month; the combination of clinical response with complete resolution of fluid collection on CT scans; the combination of granulation pink tissue in almost all walls of the cavity with significant cavity reduction on CT scans; or the combination of clinical response with pink granulation tissue on the cavity wall [41].
Regarding stent removal, as previously mentioned, it has been suggested that an assessment should be made for disruption of the main pancreatic duct before transluminal drainage is removed [9]. In case an LAMS has been used, retrieval within 4 weeks after placement is recommended [9].
Dual-Modality Drainage Technique
Combined transluminal and percutaneous drainage can be advantageous in the setting of WON extending to the paracolic gutters, given its difficult resolution with only endoscopic transmural techniques [1]. Compared to percutaneous drainage alone, the combined approach led to a shorter length of hospitalization and duration of external drainage and a lower number of drains [49]. Dual-modality drainage was also associated with a low incidence of external pancreatic fistulas [49].
Complications
Adverse events associated with EUS-guided transmural drainage can occur early during the stent deployment phase or late due to subsequent effects of the intervention. They may include bleeding (up to 18%), free perforation (up to 4%), stent maldeployment, migration, occlusion, secondary infection, aspiration of gastric content, air embolism, abdominal pain, post-procedure fever, gastric outlet obstruction, pancreatic duct damage and sedation-related complications [1, 24, 48].
Adverse events may also arise during endoscopic necrosectomy. Based on a systematic review [50], complications occurred in 36% of the patients. The most common complication was bleeding (18%). Pancreatic fistula occurred in 5%, spontaneous perforation of a hollow organ (excluding the stomach/duodenum, due to the intervention) in 4%, and air embolism in 1% of the patients.
Early recognition and management are essential to avoid long-term sequelae and poor outcomes. Therefore, endoscopic drainage and necrosectomy of PPC should be performed with the availability of surgical and interventional radiology support [1].
Key Points
• PPC are a known complication of acute pancreatitis. They can also occur secondary to pancreatic trauma or surgery, chronic pancreatitis or autoimmune pancreatitis.
• PPC are categorized into four types: (1) acute peripancreatic fluid collection, (2) pancreatic pseudocyst, (3) acute necrotic collection and (4) WON.
• Most PPC either resolve spontaneously or are persistent but asymptomatic. Intervention is needed for a minority of patients with infected or symptomatic PPC.
• EUS-guided transmural drainage is the technique of choice for PPC management.
• The procedure should be delayed, whenever possible, until at least 4 weeks after initial presentation to allow wall maturation and better necrotic tissue demarcation and liquefaction.
• Differentiation between a pseudocyst and WON is important, since the former will likely need only drainage, while the latter may need further debridement of necrotic tissue after initial drainage.
• Both metal stents and PS lead to great treatment success, although metal stents may offer some advantages.