Introduction
Drug-induced liver injury is one of the most challenging liver disorders because of the myriad of drugs used in clinical practice, the available herbs and dietary supplements with hepatotoxic potential, the ability of the condition to present with a variety of clinical and pathological phenotypes, and the current absence of specific biomarkers. This diagnosis requires a high degree of awareness of the condition and careful exclusion of alternative etiologies. Hepatotoxicity can be severe, leading to acute liver failure [1].
It is known that occupational exposures can induce liver injury in a similar way to prescription drugs; herbal and dietary supplements and workplace exposure have been implicated in the full spectrum of liver disease. A variety of chemicals, including solvents, pesticides, and metals, have been linked to occupational liver diseases [2].
Contact with these hepatotoxic agents may result from occupational exposure. Workplace exposure has been associated with acute and chronic liver diseases. However, its prevalence is inadequately quantified, and their epidemiology is limited [2].
Occupational liver diseases may result from accidental exposure to a significative quantity of product, which is uncommon and easily recognized, or from prolonged lower-level exposures, which is more frequent but often overlooked. Normally, prolonged lower-level exposures have an insidious onset and may be asymptomatic or obfuscated and confounded by concurrent conditions [2].
Isopropanol or isopropyl alcohol (2-propanol) is a volatile liquid that is miscible in both water and organic solvents. Due to its useful properties and low toxicity, isopropanol has been widely used for many years as a solvent, rubbing alcohol, and mild disinfectant. Despite the long experience with its use, the toxicological data on isopropanol has been, until recently, relatively limited [3].
Case Report/Case Presentation
A 33-year-old Caucasian female hairdresser was admitted to the hospital with fatigue, persistent epigastric pain, and jaundice, which had appeared insidiously 2 weeks before and progressively worsened. She had no fever or urine and stool alterations.
She had been taking ibuprofen 600 mg every 12 h for the last 2 months for a uterine cervix inflammatory lesion. She had a history of depression and had been medicated with sertraline for 5 years.
On physical examination the patient had jaundice, with no encephalopathy or other alterations, but no other alterations were found. Laboratory tests showed the following values: aspartate transaminase (AST), 485 U/L (>15× upper limit of normal [ULN]); alanine transaminase (ALT), 908 U/L (>20× ULN); alkaline phosphatase (ALP), 240 U/L (2 ×ULN); γ-glutamyltransferase (GGT), 370 U/L (>6 ×ULN); total bilirubin (TB), 3.5 mg/dL (>2 ×ULN); direct bilirubin, 2.1 mg/dL; albumin, 3.7 g/dL; platelets, 160 g/L; and INR value, 1.29. The abdominal ultrasound with Doppler evaluation was normal. Infectious, metabolic, immune, and vascular causes were excluded.
Liver biopsy revealed: a portal space inflammatory infiltrate with small lymphocytes and eosinophils and focal lesions of the limiting lamina; at the lobular level and predominantly in the 3 acinar zones hepatocyte necrosis with some centrocentral bridges, acidophilic bodies, and mild lymphocytic and granulocytic inflammatory infiltrate; some histiocytic and inflammatory cells in acinar zones 1 and 2; and no deposits of iron (Perls), copper (rhodanine), or hyaline blood cells (PAS-D). Acute lobular and portal hepatitis with confluent centrilobular necrosis was found (Fig. 1). These findings allowed ruling out of other etiologies such as autoimmune or metabolic causes, and a diagnosis of toxic hepatitis was assumed.
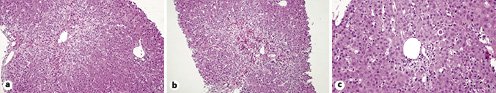
Fig. 1 Liver biopsy. a, b HE. ×100. Lobular region (acinar zone 3) with lymphocytic and granulocytic inflammatory infiltrate, with necrotic hepatitis and centrocentral bridges. c HE. ×200. Portal space with lymphocytic inflammatory infiltrate and focal lesions on the limiting lamina, adjacent to an acidophilic body.
Ibuprofen intake was suspended, and she was kept only on symptomatic therapy. The patient slowly improved and at discharge she was asymptomatic and analytical changes were progressively normalizing; the following values were as follows: AST, 324 U/L; ALT, 521 U/L; ALP, 234 U/L; GGT, 330 U/L; TB, 2.9 mg/dL; and direct bilirubin, 1.3 mg/dL, with normal albumin (4.2 g/dL), platelet counts (215 G/L), and INR (i.e., 1.15). The existence of an ibuprofen-induced liver injury was assumed. During the initial follow-up period, analytical values completely normalized.
After returning to her job, she complained of extreme fatigue and, analytically, there was a new worsening, with the following values: AST (289 U/L), ALT (602 U/L), ALP (159 U/L) and GGT (186 U/L) elevation, TB 1.4 mg/dL, direct bilirubin 0.8 mg/dL, and INR 1.13, despite ibuprofen withdrawal. By that time, an occupational exposure was suspected and indeed corroborated after complete clinical and analytical recovery with professional activity suspension and home rest.
Professional toxic chemicals exposure was assessed. It was concluded that the symptoms settled after regular usage of a new permanent hair-straightening product containing isopropanol. Our patient was exposed to this new product on a daily basis. She resumed her craft banning this product and no recurrence was seen after more than 1 year of follow-up.
Discussion/Conclusion
In the last 30 years, experimental tests have been conducted to evaluate the pharmacokinetics and toxicity of isopropanol. The goal of these tests is to provide the experimental basis for a more confident evaluation of potential human health risks from isopropanol exposure [3].
It is used in body rubs, hand lotions, aftershave lotions, cosmetics, and pharmaceuticals, as well as in antifreeze, industrial solvent, solvent for gums, essential oils and resins, quick-drying inks, and antiseptic and pharmaceutical aids.
Toxic effects have been described in occupational and accidental exposures and include central nervous system depression and liver, kidney, cardiovascular, and brain damage. The absorption can occur by oral intake and dermal or inhalation exposure [4]. In elevated concentrations, particularly ingestion, isopropanol can be lethal.
In the EASL Clinical Practice Guideline, acute liver injury is defined by the presence of any one of the following criteria: ALT ≥5× ULN, ALP ≥2× ULN (particularly if there is concomitantly elevated GGT in the absence of bone disease), and ALT ≥3× ULN and simultaneous TB >2× ULN. Besides that, liver injury can be classified as hepatocellular, cholestatic, and mixed pattern according to the R value ([ALT/ULN]/[ALP/ULN]) [2].
These definitions allow the identification of an acute hepatic reaction in people exposed to hepatotoxins [2].
The diagnosis of an occupational liver disease relies on a high level of suspicion and there are no pathognomonic signs. Occupational history is essential, and the work environment must be explored, considering that occupational exposure occurs usually by inhalation and through the skin [2].
In our case, the existence of a temporal relationship with the administration of the drug, resolution after withdrawal of the drug, recurrence of the adverse effect after reexposure, and the absence of another alternative cause allowed the diagnosis of isopropanol hepatotoxicity. The patient was exposed to isopropanol by inhalation. Alveolar concentration is correlated to the environmental concentration at any given time. The inhalation exposure to isopropanol varies from 0.4 to 14.8 mg/m3, with a mean of 5.6 mg/m3, for hairdressers in Norway [5].
After absorption, isopropyl alcohol is distributed in body water and 20-50% is excreted unchanged. Most of it is metabolized in the liver by alcohol dehydrogenase into acetone, formate, and finally carbon dioxide [4]. It is metabolized at a slower rate than ethanol [6]. Acetone is gradually eliminated by the kidneys (∼60%) or lungs (∼40%). Clinically insignificant excretion occurs into the stomach and saliva [4].
Isopropanol can cause liver damage itself or increase other drugs’ hepatotoxicity. The association between occupational exposure and liver injury has been shown. Lavicoli et al. [7] developed a study where they measured the serum levels of ALT, AST, and GGT in 40 printer workers exposed to isopropanol, and they concluded that the serum levels of ALT, AST, and GGT were higher in the exposed workers than in the nonexposed ones. The results of this study showed that the removal of isopropanol from the industry had a positive health effect, improving the hepatic function of the workers [7]. Even isopropanol in small concentrations, particularly in prolonged exposures, can cause damage [8].
In our case, the diagnosis was not easy but, in order to establish causality, a coherent synthesis was made between the characteristics of the patient’s disease, the exclusion of other liver disorders, collection of a thorough occupational history, the presence of hepatotoxic chemicals, and their known capability to cause that disease. Besides that, we noted recurrent acute liver injury episodes, occurring after reexposure to isopropanol at work, and resolution of symptoms and normalization of blood tests after exposure cessation.
In conclusion, there are no specific biomarkers for the diagnosis and no specific treatment. A high clinical suspicion is essential for the diagnosis. Treatment is symptomatic and decontamination guaranteed.














