Introduction
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 12, 2020. Its causative agent, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is now responsible for over 32 million confirmed cases and almost 1 million documented deaths in all continents [1]. COVID-19 can range from a self-limited disease to a severe pneumonia with high mortality rates. It is estimated that gastrointestinal symptoms can be seen in up to 10% of patients with COVID-19 [2]. The impact of SARS-CoV-2 infection on the liver and the possibility of chronic liver disease (CLD) as a risk factor for the severity of COVID-19 has been studied and it is still unclear [3, 4]. Liver function test abnormalities, indicated by elevations of liver aminotransferases, total bilirubin and prolonged prothrombin time have been reported [5-10]. The impact of abnormal liver tests in patient outcome is controversial. Some reports have shown that patients who developed abnormal liver tests had longer hospitalization lengths [8] and were at a higher risk of progressing to severe disease [11]. In opposition, other recent studies argue that the COVID-19-related liver injuries are usually transitory and mild, with small clinical significance [12]. Conflicting data exist concerning the clinical characteristics of liver failure among patients with COVID-19. The etiology of such alterations could be explained by several factors like the virus itself, hypoxic hepatitis, hepatic congestion due to mechanical ventilation, drug toxicity, immune response and alterations in gut vascular barrier and microbiota [13, 14]. Scarce pathology data have been reported so far, which makes it difficult to address this issue, with some studies reporting coronavirus particles in hepatocytes’ cytoplasm which might point to a direct effect induced by viral infection [11]. There is still scarce information regarding the global prevalence and etiology of pre-existing CLD in individuals with COVID-19, but it has been estimated to be relatively low (around 3%). When reported, the main cause of chronic liver disease was attributed to hepatitis B and C virus infection [15]. Some studies report that patients with pre-existing CLD may have increased susceptibility to more severe liver damage, more severe COVID-19 and higher mortality from COVID-19 [16, 17]. Cases of acute-on-chronic liver failure have been reported, supporting this hypothesis [18]. We describe the clinical outcomes of patients with SARS-CoV-2 infection regarding the presence of abnormal liver tests and CLD.
Materials and Methods
Patients and Study Design
We performed a retrospective analysis of a cohort of consecutive adult patients hospitalized for SARS-CoV-2 infection in the Centro Hospitalar Universitário do Porto from March 2 to May 4, 2020. Patients with nosocomial SARS-CoV-2 infection were also included. The diagnosis of SARS-CoV-2 infection was done according to WHO guidelines [19], by a positive result of reverse transcription polymerase chain reaction (RT-PCR) in nasopharyngeal swab specimens. Hospitalization criteria were those defined by the Direção Geral da Saúde (Directorate-General for Health in Portugal). During the containment phase, up to March 26, all positive patients were hospitalized, including asymptomatic patients. From that date on, during delay and mitigation phases, only patients which fulfilled the established criteria were hospitalized [20].
This study was approved by the Ethics Committee of the Centro Hospitalar Universitário do Porto (2020.087[070-DEFI/071-CE]).
Data Collection and Patient Management
Clinical, analytical and imaging data were collected from electronic medical records. Laboratory testing was performed according to clinical care needs and consisted of complete blood count, blood chemical analysis, coagulation testing, assessment of liver and renal biochemistry, measures of ions, C-reactive protein, procalcitonin, lactate dehydrogenase, myoglobin and creatine kinase. Radiological assessments included chest radiography and/or computed tomography (CT).
All patients received supportive treatments. Because there was no approved antiviral treatment at the time, some patients were treated with hydroxychloroquine, lopinavir/ritonavir or remdesivir with no standard guidance on drug choice. Antibiotics and corticosteroids were used based on the need and health care providers’ discretion.
Study Outcomes
Studied outcomes were disease duration, hospitalization length, COVID-19 severity, admission to an intensive care unit (ICU) and mortality. These outcomes were analyzed by the presence of abnormal liver tests, chronic liver disease or liver cirrhosis.
Study Definitions
Alcohol consumption was classified as low to moderate if regular alcohol consumption was inferior to 20 g/day in females and 30 g/day in males and heavy if superior doses were registered. CLD was considered based on medical records. Cirrhosis was considered in patients with clinical features and imaging/endoscopy suggestive of CLD and portal hypertension, or a previously diagnosed case of cirrhosis. Child-Turcotte-Pugh and Model of End-Stage Liver Disease-Sodium scores were calculated for patients with liver cirrhosis [21, 22]. Respiratory failure was defined as mild (PaO2/FiO2 200-300 mm Hg), moderate (100-200 mm Hg) and severe (<100 mm Hg). Acute kidney injury was staged according to KDIGO criteria [23]. Pneumonia severity was evaluated according to lung involvement extension in chest CT as mild (up to 3 spots of ground glass and each spot <3 cm), moderate to severe (more than 3 spots of ground glass, presence of a focus of more than 3 cm or ground glass with small consolidations) or severe (diffuse ground glass or architecturally distorted consolidations).
Disease duration was defined as the interval between the onset of symptoms and virus clearance confirmation - 2 negative RT-PCR results on sequential samples taken at least 24 h apart - or death, whichever came first. COVID-19 severity was classified as mild (presence of constitutional and/or upper respiratory tract symptoms with none or mild changes on chest radiography or CT), moderate to severe (lower respiratory tract symptoms and evidence of pneumonia and/or sign of respiratory failure such as dyspnea, respiratory rate >30 cpm, pulse oximetry ≤92% or PaO2/FiO2 <300 mm Hg) or critical (severe pneumonia and respiratory or another organ failure requiring ICU admission).
Liver test abnormalities were defined as any serum concentration of the following parameters above the upper limit of normal value (women/men): aspartate transaminase (AST) >30/34 U/L, alanine transaminase (ALT) >36/44 U/L, alkaline phosphatase (ALP) >104/129 U/L, γ-glutamyl transferase (GGT) >39/66 U/L and total bilirubin >1.00 mg/dL.
Statistical Analysis
Categorical variables were described as frequency and percentages, and continuous variables as mean and SD or median and interquartile range (IQR), as appropriate. Means for continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann-Whitney U test was used. Comparison of categorical variables was done using the χ2 test or the Fisher exact test, if the cell counts were small. Variables with a p value <0.1 in univariate analysis were included in the multivariable analysis (binary logistic regression).
Statistical analysis software IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used for all analyses in this study. A p value <0.05 was regarded as statistically significant.
Results
Clinical Characteristics of Patients with COVID-19
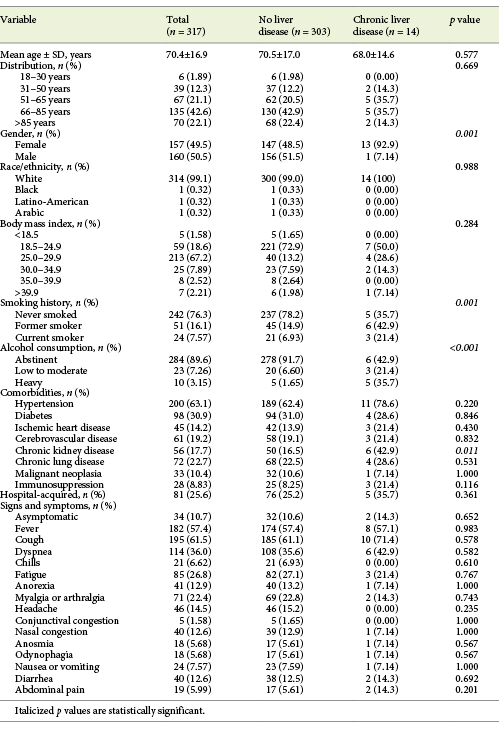
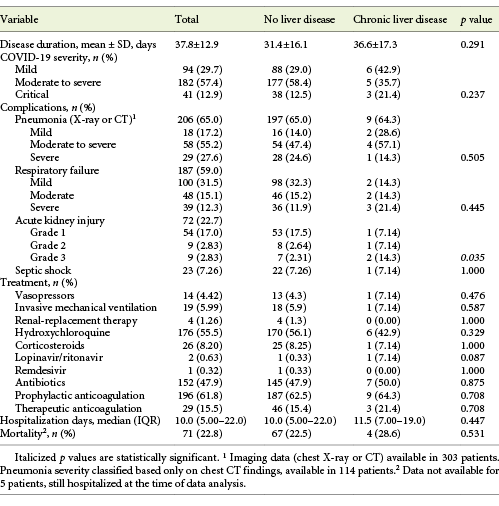
A total of 317 inpatients with SARS-CoV-2 infection were included, with a mean age of 70.4 ± 16.9 years (22.1% over 85 years) and 50.5% males. COVID-19 severity was moderate to severe in 57.4% and critical in 12.9% of the patients. The mean disease duration was 37.8 ± 12.9 days, the median hospitalization length 10.0 days (IQR 5.00-22.0) and overall mortality rate 28.6% of the patients. Hospital-acquired SARS-CoV-2 infection was registered in 81 patients (25.6%). Further clinical characteristics of patients with COVID-19 can be found in Tables 1-3.
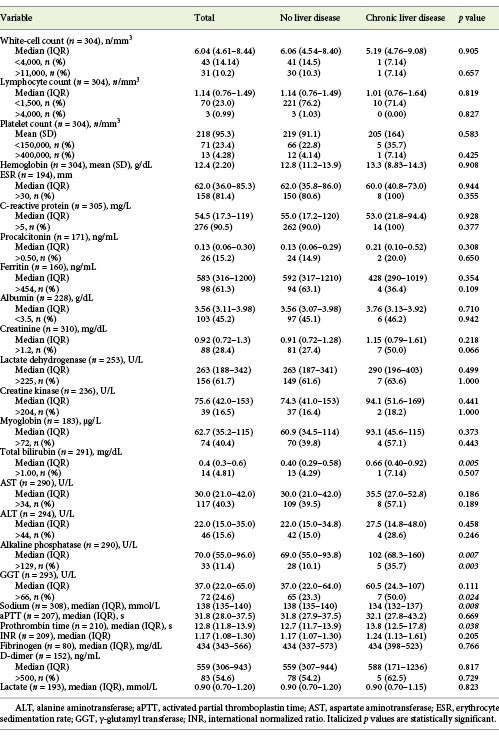
Table 2: Laboratory data at admission of patients with COVID-19, with or without chronic liver disease
Clinical Characteristics of Patients with COVID-19 and Abnormal Liver Tests at Admission
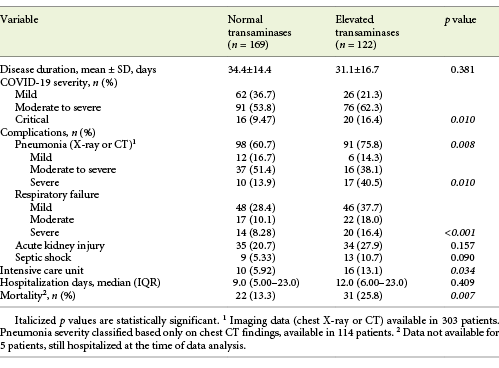
At admission, about half of the patients (n = 148/294, 50.3%) showed abnormal liver tests, and 122 (41.5%) patients showed elevated aminotransferase levels, from which the majority (75.4%) were mild elevations of 1-2× the upper limit of normal (online suppl. Table 1, see www.karger.com/doi/10.1159/000513593). AST levels were elevated in 117 (40.3%; median 46.0 U/L, IQR 39.0-67.0) and ALT levels in 46 (15.6%) patients (median 70.0 U/L, IQR 53.0-87.0). ALP was elevated in 33 (11.4%; median 167 U/L, IQR 145-231) and GGT in 72 (24.6%) patients (median 105 U/L, IQR 84.8-154). Total bilirubin was abnormal in only 14 (4.81%) of the patients.
From baseline clinical characteristics, only male gender (62.3 vs. 37.3%, p = 0.001) and age >65 years (71.3 vs. 28.7%, p = 0.006) were associated with elevated aminotransferase levels. Presence of any COVID-19 symptom was also associated with elevated aminotransferase levels compared to asymptomatic patients (p = 0.005). When both groups were compared, a difference for frequency of symptoms was seen for dyspnea, headache and anorexia (Table 4). Patients with hospital-acquired COVID-19 showed a lower frequency of aminotransferase elevation (17.4 vs. 29.0%, p = 0.022).
Table 4: Clinical characteristics of patients with COVID-19, by aminotransferase levels at admission
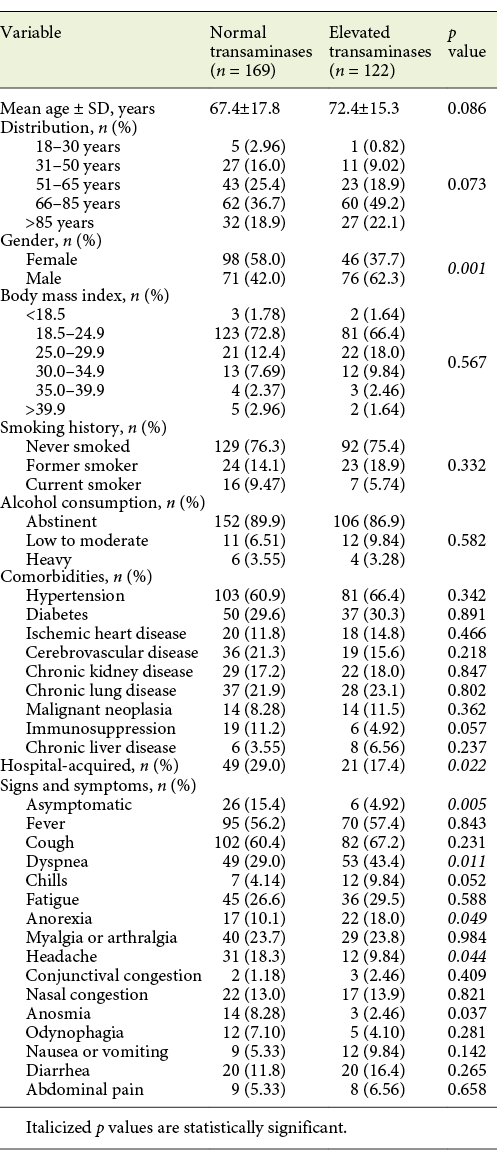
Elevated aminotransferase levels (either AST and/or ALT) at admission were associated with COVID-19 severity (p = 0.01), presence of pneumonia (p = 0.008) as well as its severity (p = 0.01) and respiratory failure severity (p < 0.001). Elevated aminotransferases were not associated with COVID-19 disease duration or hospitalization days. They were associated, though, with ICU need (13.1 vs. 5.92%, p = 0.034) and with increased mortality (25.8 vs. 13.3%, p = 0.007; Table 5).
In multivariate analysis, only male gender (OR 5.13; 95% CI 1.48-17.9; p = 0.01) and respiratory failure (OR 9.30; 95% CI 1.42-61.1, p = 0.02) were associated with elevated aminotransferases.
When performing subgroup analyses for COVID-19 outcomes, we concluded that elevated ALT at admission was associated with ICU admission (34.6 vs. 13.8%, p = 0.005), but not mortality, COVID-19 severity, disease or hospitalization length. By contrast, AST was associated with pneumonia development (46.0 vs. 30.1%, p = 0.011) and its severe forms (59.3 vs. 26.7%, p = 0.002), with respiratory failure (51.2 vs. 25.8%, p < 0.001) and its severity (p < 0.001), moderate to critical COVID-19 (45.5 vs. 28.4%, p = 0.006) and mortality (58.5 vs. 36.2%, p = 0.003), but not with disease or hospitalization length or ICU admission.
On the other hand, elevated ALP was associated with critical COVID-19 (21.1 vs. 9.92%, p = 0.044) and with mortality (20.4 vs. 9.52%, p = 0.025) while elevated GGT was associated with ICU admission only (42.3 vs. 22.8%, p = 0.028).
Characterization of Patients with CLD and COVID-19
In our cohort a prevalence of 4.42% of CLD was found, with alcohol (n = 6) and nonalcoholicic fatty liver disease (NAFLD)/steatohepatitis (n = 6) being the most frequent etiologies (Table 6). From these patients, 3 had liver cirrhosis.
Patients with CLD were more frequently men (p = 0.001), current or former smokers (p < 0.001) and alcohol consumers (p < 0.001). Chronic kidney disease was also more frequent among CLD patients (p = 0.011). All other baseline characteristics were similar between the 2 groups (Table 1). Overall, there was no difference in COVID-19 symptoms (Table 1), laboratory data at admission (Table 2), complications and treatments between patients with or without CLD (Table 3). Exceptions were seen for higher levels of ALP (102 vs. 69.0 U/L, p = 0.007), lower levels of serum sodium (134 vs. 138 mmol/L, p = 0.008) and higher prothrombin time (13.8 vs. 12.7 s, p = 0.038) for CLD patients. Also, more severe degrees of acute kidney injury during hospitalization were found in these (21.4 vs. 4.95%, p = 0.035).
COVID-19 patients with CLD showed an increased frequency of critical COVID-19 (21.4 vs. 12.5%, p = 0.237), a longer disease (36.6 vs. 31.4 days, p = 0.291) and hospitalization length (11.5 vs. 10.0 days, p = 0.447), as well as an increased mortality rate (28.6 vs. 22.5%, p = 0.595; Table 3). When the subgroup of cirrhotic patients was analyzed, a longer inpatient length of stay (15 vs. 10 days, p = 0.639), an increased frequency of moderate to severe and critical COVID-19 (66.6 vs. 54.6%, p = 0.844) and an increased mortality rate (66.7 vs. 18.2%, p = 0.176) were seen.
Discussion/Conclusion
The state of emergency in Portugal due to SARS-CoV-2 pandemic infection was declared on March 18, 2020. During this time reorganization of hospitalization units was done and “COVID-19 units” were created to receive patients infected with SARS-CoV-2. We hypothesized the existence of differences in the SARS-CoV-2 infection course in patients with abnormal liver tests and between patients with and without CLD. In this study we analyzed the data regarding a cohort of 317 patients admitted to COVID-19 units in a tertiary university-teaching hospital in Portugal, during a 10-week period. Apart from acute respiratory syndrome induced by COVID-19, several studies report liver enzyme abnormalities in up to 50% of SARS-CoV-2-infected patients, at admission, even before the use of various potential hepatotoxic drugs [3]. Accordingly, we have shown abnormal liver tests at admission in 50.3% of the patients in our cohort. Moreover, elevated aminotransferase levels (AST and/or ALT) were seen in 41.5% of our patients, mostly in males and patients older than 65 years. Male and older patients with COVID-19 were previously identified has having a higher risk of liver injury [24]. Despite the fact that most of these elevated aminotransferase levels (75.4%) were mild, up to 2× the upper limit of normal, they still were associated with worse clinical outcomes such as respiratory failure, severe pneumonia, moderate to severe and critical COVID-19, ICU admission and increased mortality. However, in multivariate analysis, all these outcomes lost their statistical significance, meaning they were probably confounders. The literature remains controversial on whether liver enzyme abnormalities truly mean worse clinical outcomes or are simple bystanders. Previous reports have associated abnormal liver enzymes with longer mean hospital stay [6-11, 18, 25-31], COVID-19 severity [6, 10, 11, 24-29, 32-34], ICU admission [7, 29] and mortality [9, 24, 34], whereas others failed to show worse clinical outcomes [7, 18, 25, 28, 30, 31]. Different mechanisms have been proposed to explain liver test abnormalities such as direct viral hepatotoxicity, drug-induced liver injury, systemic effects of viral infection and sepsis, or exacerbation of an underlying liver disease [35]. Although laboratory testing was done at admission, before inpatient drug administration and thereby eliminating any potential hepatotoxic effect as a confounder, no records of usual outpatient medication were available. The alterations on aminotransferase levels at admission could reflect the presence of a subset of patients with unrecognized CLD, in accordance with the retrospective nature of our analysis. Given the low prevalence of CLD patients in our cohort, not different from other studies, this possibility is unlikely to justify the observed alterations in liver test abnormalities. The association seen in our study of aminotransferase elevation with respiratory failure is compatible with hypoxic hepatitis as possible contributing factor to liver damage. Also, not negligible, lactate dehydrogenase, creatine kinase and myoglobin were elevated in 61.7, 16.5 and 40.4% of our patients, respectively, raising the suspicion that aminotransferase elevations do not necessarily arise from the liver alone and that COVID-19 infection might induce a myositis similar to that observed in severe influenza infections [13]. Corroborating this hypothesis is the fact that, in a subgroup analysis, only AST elevation, and not ALT elevation, was associated with respiratory failure.
Relevant elevations of ALP and GGT levels have not been reported in most studies [3]. In our work, we found elevated ALP levels in 11.4% and elevated GGT levels in 24.6% of the patients, similar to the previously reported prevalences of 3.91-13.8% and 18.2-40.3%, respectively [6, 36, 37]. Both enzymes are present in several extrahepatic tissues, although for bile duct injury, ALP is considered more sensitive than GGT [6]. Hence, for patients with elevated GGT and normal ALP, other etiologies apart from liver damage should be considered. In our study, we also found ALP to be associated with critical COVID-19 and mortality, contrary to what has been previously reported [37, 38]. Only one trial has previously shown ALP association with severe COVID-19 [27], but none, apart from our study, has shown an association with mortality.
Nosocomial SARS-CoV-2 infection has been underreported in previous studies. In our cohort, we had 25.6% of the patients with hospital-acquired SARS-CoV-2 infection. Surprisingly, these patients showed a lower frequency of aminotransferase elevation (17.4 vs. 29.0%, p = 0.022) which may reflect a selection bias, since 44.4% of nosocomial COVID-19 demonstrated mild disease (in contrast to 24.3% of “community-acquired” COVID-19) and probably would not need hospitalization for COVID-19 reasons.
The relationship between the presence of CLD and COVID-19 is a matter of debate bearing in mind the putative influence of cirrhosis-associated immune dysregulation and immunosuppressant use, together with the role of immune processes in SARS-CoV-2 infection pathology [4, 39]. Two systematic reviews exploring the relationship between liver disease and COVID-19 failed to show an increased risk of COVID-19 progression in patients with previously known CLD [34, 40]. On the other hand, the presence of CLD is believed to be a risk factor for a severe COVID-19 course, in spite of the lack of data relating both [3]. Due to the difficulties in identification of pre-existing liver conditions in COVID-19 patients, the mutual impact of CLD and COVID-19 has been difficult to analyze. The prevalence of CLD in patients infected with SARS-CoV-2 has been reported between 0.6 and 37.6% [3]. In our cohort we showed a prevalence of CLD (4.41%) slightly superior to that shown by recently published meta-analyses [15, 24, 34]. Mantovani et al. [15] made a meta-analysis of 11 observational studies capturing 2,034 Chinese adults with COVID-19, with 62 presenting a prior history of CLD with an overall prevalence of CLD of 3%. In this analysis, the largest so far to our knowledge regarding the prevalence of CLD in COVID-19 inpatients, the main etiology of CLD was due to viral infections (HBV and HCV) with most patients being young males. Our subgroup of patients with CLD was mainly composed of male patients with a mean age of 68 years, with cardiovascular risk factors, comorbidities such as chronic kidney and lung disease, and previous or ongoing smoking and alcohol-drinking habits. The presenting signs and symptoms of this subgroup did not statistically differ from those without CLD. We have also shown significant differences for this subgroup of patients on some laboratory abnormalities, with known prognostic relevance, such as lower sodium levels at admission and a higher degree of kidney injury during hospitalization. Some clinically relevant outcomes regarding COVID-19 were also seen in our CLD patients, such as increased frequency of critical disease (p = 0.0237), longer disease duration (p = 0.291) and hospitalization length (p = 0.447), as well as increased mortality (p = 0.531). However, the magnitude of these differences did not reach statistical significance.
Data regarding mortality for patients with CLD and COVID-19 are still scarce, but several studies point out more severe COVID-19 and higher mortality from COVID-19 in CLD patients and particularly in cirrhotic patients [16, 17]. Moon et al. [41] published the preliminary results of international registries (COVID-Hep.net and COVIDCirrhosis.org) regarding 151 patients with CLD (of which 109 with cirrhosis) from 21 countries. This population was predominantly male, with a median age of 61 years, and with NAFLD and alcohol-related liver disease pointed out as the main etiologies for the CLD. Overall, the mortality rate for patients with CLD was 30.92% (26.97% in cirrhotic cases), higher rates than those reported in cirrhotic patients infected with influenza virus in the pre-COVID-19 era [41, 42]. This registry also showed, between survivors and nonsurvivors, significant differences in baseline values for prothrombin time, bilirubin, albumin and creatinine. Like Moon et al. [41], the main etiologies found for CLD in our cohort were NAFLD and alcohol-related liver disease and, from those who presented cirrhosis, worsening ascites with spontaneous bacterial peritonitis were the most frequent decompensation events. From these, evolution to acute-on-chronic liver failure was observed in 2 patients. When a subanalysis for cirrhotic patients compared with noncirrhotic CLD patients was made, no statistically significant difference was found for disease duration, severity of COVID-19, hospitalization time and mortality. Although a small number of patients present CLD in our cohort, their mortality rate (28.6%) was comparable to that shown by Moon et al. [41], which is in accordance with the similar demographic characteristics and liver disease etiology shared by our population and that included in this international registry. The relative higher mortality rate found for the 3 cirrhotic patients in our cohort (66.7%) can be explained by the development of critical COVID-19 associated with coexistence of multiple organ failure/acute-on-chronic liver failure. The apparently conflicting mortality data shown in the APCOLIS Study, an international registry from 13 Asian countries regarding patients with CLD and confirmed COVID-19, which has shown lower mortality rates of 2.7% among CLD patients and 16.3% among cirrhotic patients, are probably explained by different patient selection criteria. While in this international registry all patients were considered and only 13.6% had severe COVID-19, we have only included hospitalized patients and we had a higher proportion of moderate, severe and critical COVID-19 (70.3%). The small number of cirrhotic patients in our cohort might be explained by some limitations of our study such as the high dependence on medical records to determine the presence of CLD, admitting that probably the number of patients with CLD was higher. Also, an accurate protocol for measure and/or screening for CLD at admission was not done. The compliance to EASL-ESCMID and AASLD guidelines [4, 43], regarding avoidance of contact with SARS-CoV-2 hotspots for CLD patients, could reflect some bias on the number of patients presenting to the emergency room. The low prevalence of patients with CLD shown by us and other authors [13, 15, 24, 25, 34, 44], reinforces that abnormalities related to liver function seen in COVID-19 might be attributed to the systemic inflammatory response directed to the virus instead the presence of an unknown CLD.
This study has some limitations, starting with the small number of patients with CLD, limiting statistical power. Also, the study population included patients from a single institution, most of them within Porto metropolitan area, and Caucasians, so these findings cannot be generalized. Additionally, data were collected retrospectively from electronic medical records that were not standardized, leading to heterogeneity and potentially incomplete clinical reports.
Nevertheless, this is the first published cohort of patients with SARS-CoV-2 infection in Portugal and the first reporting the impact of abnormal liver tests and chronic liver disease on patient outcomes.
In conclusion, we showed liver enzyme abnormalities in COVID-19 patients were frequent but most commonly mild. AST, but not ALT, was associated with worse clinical outcomes, such as respiratory failure, pneumonia, COVID-19 severity and mortality, probably indicating these outcomes were independent of liver injury. Also, 4.41% of our cohort presented CLD and a clear impact on COVID-19 outcomes was not seen.