Introduction
Biliary leaks can be a severe surgical complication and are the most commonly reported postoperative biliary injury, particularly after cholecystectomy [1-3].
Iatrogenic injury to the biliary tree, namely, biliary leaks, occurs after laparoscopic cholecystectomy in 0.3-2.7% of patients [1, 4]. The incidence of biliary leaks in the era of laparoscopic approaches is significantly higher than for open cholecystectomy and remains relatively unchanged since the first studies, despite well-known training and technological improvements [2, 5]. Additionally, bile leaks related to laparoscopy are often more complex and difficult to treat than those that occur after open cholecystectomy [5]. There are several associated risk factors that increase the probability of a leak during surgery: (a) the existence of stones in the biliary tree after cholecystectomy can increase the pressure in the biliary system and therefore facilitate the development of a bile leak [1, 4]; (b) in the presence of severe inflammation and fibrosis related to acute cholecystitis, the use of a laparoscopic technique makes cholecystectomy more difficult and the risk of incomplete gallbladder resection and injuries even higher [6].
The management of biliary leaks has evolved from surgery into a minimally invasive procedural approach, namely, an endoscopic technique designated as endoscopic retrograde cholangiopancreatography (ERCP). This endoscopic technique diagnoses biliary injury and, when a leak exists, decreases or eliminates the pressure gradient between the bile duct and the duodenum, thus creating a preferential transpapillary bile flow and allowing the leak to seal [1, 3, 6].
The outcome of leak closure can be accomplished by a variety of endoscopic interventions, including biliary stenting or nasobiliary drainage, with or without sphincterotomy, or biliary sphincterotomy alone [1-4, 6]. However, biliary leaks have different grades of severity and complexity, and there have been reports of leaks being treated with multiple endoscopic procedures, or even endotherapy failures for which surgery is required [1, 3, 6]. Due to the variety of leak complexities and available endotherapy modalities, no clear strategy for endotherapy has emerged and, most of the time, treatment is provided on an individual basis. Furthermore, ERCP is associated with serious and not infrequent adverse events. Therefore, the definition of the criteria, timing, and strategy for biliary drainage in the face of a suspected leak is paramount [2, 7, 8]. In this article, we review the types of postsurgical biliary leaks and endoscopic treatment findings.
Types and Classification of Biliary Injuries
Iatrogenic injury of the biliary tree is associated with several types of lesions, namely, strictures (early or late and of minor or major severity), leaks, and transections. Bile duct injuries may have different causes, such as clipping, burning, direct damage, and cutting, and are not uncommonly associated with vascular injuries, most frequently to the right hepatic artery [5].
There are several classifications of iatrogenic biliary injuries, which differ in aim, scope, and complexity.
The first classification of biliary injuries was proposed by Bismuth and Majno [9] in 1982, and it mimics Bismuth’s classification for hilar tumors. The Bismuth classification for biliary injuries is simple, based on the location of the injury and is very helpful after repair: type I, located ≥2 cm from the main hepatic confluence; type II, located <2 cm from the main hepatic confluence; type III, affecting the hepatic confluence, which is patent; type IV, involving hepatic confluence and interrupting it; and type V, when an aberrant right hepatic duct is injured, with or without injury of the CBD [5]. The strength of Bismuth’s classification is its simplicity and similarity to the widespread classification of hilar tumors. However, it does not include the whole spectrum of biliary injuries.
In 1995, Strasberg et al. [10] proposed a wider classification for iatrogenic biliary injuries (Fig. 1), which is useful for classifying complex cases in which a biliary leak is associated with other bile duct injuries [5]. They considered 5 types of injuries, A-E, and type E is subdivided into 5 subtypes. Type A corresponds to a bile leak from a cystic duct stump or Luschka’s duct leaks, type B is an occluded right posterior sectorial duct, and type C is a bile leak from the divided right posterior sectorial duct. In type D, there is a bile leak from the main bile duct involving <50% of its circumference. In type E1, a transected main bile duct with a stricture >2 cm from the hilum is observed; in type E2, this stricture is located <2 cm from the hilum. In type E3, there is a stricture of the hilum with right and left ducts in communication; in type E4, this stricture separates the right and the left ducts. In type E5, there is a stricture of the main bile duct plus a stricture of the right posterior sectorial duct. Types E1-E5 correspond to Bismuth types I-V. Strasberg type A injuries account for up to 85% of all cases (75% are cystic duct stump leaks and 10% are leaks from the ducts of Luschka) [10].
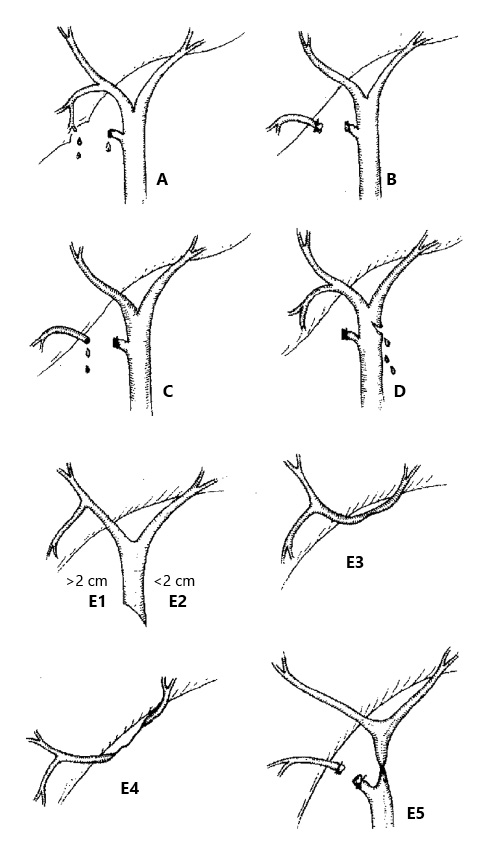
Fig. 1: Strasberg classification of biliary injuries. Type A: bile leak from a cystic duct stump or Luschka’s duct leaks. Type B: occluded right posterior sectorial duct. Type C: bile leak from divided right posterior sectorial duct. Type D: bile leak from the main bile duct involving <50% of its circumference. Type E1: transected main bile duct with a stricture of >2 cm from the hilum. Type E2: stricture located <2 cm from the hilum. Type E3: stricture of the hilum with right and left ducts in communication. Type E4: stricture separating the right and left ducts. Type E5: stricture of the main bile duct plus a stricture of the right posterior sectorial duct.
One of the most popular classifications when biliary leaks are the predominant injury is the Amsterdam classification, which defines 4 types of injuries sustained during laparoscopic cholecystectomy [11]: type A, leakage from the cystic duct or peripheral radicals; type B, major bile duct injury with leakage; type C, bile duct stricture without leakage; and type D, complete transection or excision of the common bile duct.
Other classifications have been described, namely, the McMahon, Stewart-Way, and Hannover classifications, but these have no significant advantages in clinical practice [12-14].
In 2004, another definition was added to the biliary leak lexicology. In a retrospective review of 207 patients, Sandha et al. [2]proposed a two-category system for grading bile leak severity, i.e., low-grade (LG, i.e., a leak identified only after opacification of the intrahepatic biliary duct) and high-grade (HG, i.e., a leak observed fluoroscopically before intrahepatic opacification. In their original report, the authors associated HG leaks with the need for more aggressive therapy (a combination of biliary sphincterotomy and the placement of a transpapillary biliary stent) or worse outcomes (specifically, the need for placement of >1 stent or even surgery).
Here, we will also refer to biliary leaks as LG or HG, and as simple or complex if they are associated with other injuries, namely, biliary strictures [2].
Endoscopic Management of Biliary Leaks
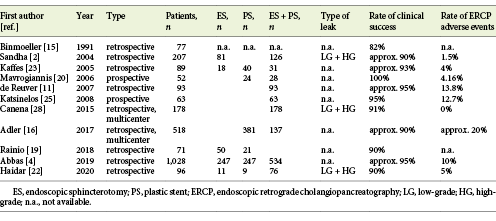
ERCP has emerged as the primary treatment for biliary leaks. Binmoeller et al. [15], in 1991, first reported the endoscopic management of postoperative biliary leaks. In their original report, endoscopic treatment was technically successful in 95% of cases and resulted in biliary leak healing in 82%. Not surprisingly, they found that cystic stump leaks had a better prognosis for healing than common bile duct or hepatic duct leaks. Since then, and for a period of 30 years, published data have suggested a success rate of ≥90% for leak closure (Table 1), currently rendering ERCP the first-line treatment for biliary leaks [1-5, 11, 16-18]. Several methods of endotherapy have been reported, and there is clearly conflict regarding the clinical success of sphincterotomy, stenting, or a combination of the two. Other conflicting issues are: (a) small- versus large-diameter stents, (b) appropriate endotherapy for HG versus LG leaks, and (c) how to treat refractory biliary leaks. Finally, controversy about the timing of endotherapy and stent retrieval remains. All of these issues are highlighted in this review.
Timing of Endoscopic Intervention
There is some controversy about the timing of endoscopic intervention. From the theoretical point of view, late treatment of biliary leaks can lead to serious adverse events and a higher mortality rate. However, there is no consensus about the appropriate timing of endotherapy for biliary leaks, and different centers recommend different time points for performing ERCP. Some centers consider biliary leakage to be an emergency whereas others treat it as a nonemergent procedure [16, 17].
The first study designed to investigate the relationship between the timing of endotherapy for a bile leak and the clinical outcomes was performed by Adler et al. [16]in 2017. It was a multicenter, retrospective study including 518 patients from 2 academic medical centers who underwent ERCP following a biliary leak (70% postcholecystectomy). The timing of ERCP was as follows: within the first 24 h, on day 2 or 3, or after 3 days. There was no significant difference in the rate of adverse events after the ERCP based on this timing. However, the 90-day mortality rate was lower in the group who underwent ERCP on day 2 or 3 than in those admitted for endotherapy within 1 day or after 3 days, but this difference was not statistically significant.
In 2019, Abbas et al. [4] published a retrospective, nationwide, inpatient analysis of 1,028 patients who underwent ERCP for a bile leak. As in Adler et al. [16], ERCP was classified as an emergency, urgent, and expectant if it was performed within 1 day, after 2 or 3 days, or after 3 days after the biliary leak occurred, respectively. The ratze of post-ERCP adverse events (defined as requiring pressor infusion, endotracheal intubation, invasive monitoring, or hemodialysis) were 11, 10, and 9% for emergency, urgent, and expectant ERCP, respectively, with no statistically significant differences. However, also similar to Adler et al. [16], they showed an in-hospital mortality with a U-shaped trend of 5, 0, and 2% for emergency, urgent, and expectant ERCP, respectively (p < 0.001). The authors suggested that this finding may have resulted from a severity bias, i.e., that patients with greater initial clinical severity were more likely to undergo emergency ERCP or a late procedure until clinical stabilization. Obviously, the clinically stable patients (with a theoretical better prognosis) received a planned ERCP within 2 or 3 days. Both studies concluded that the timing of ERCP was not a significant predictor of post-ERCP adverse events, suggesting that ERCP can usually be performed in an elective rather than an urgent manner.
In the face of the dedicated available literature, offering a clear recommendation is not possible. However, and according to existing evidence, interventions for biliary leaks should not be delayed for longer than 3 or 4 days, especially if there is no evidence of reduction in the daily volume of a percutaneous drain output or if there is a clinical worsening scenario. Furthermore, there is also no clear indication for urgent intervention, except in cases where a large leak is documented or inferred by high volume drainage. Therefore, the suggestion emerging from the literature is that endoscopic intervention should take place on day 2 or 3 after a persisting biliary leak is diagnosed, although this is not a strong recommendation and the evidence is weak.
Sphincterotomy, Stenting, and Combination Therapy
Data from different studies have been used for comparison. In a trial from Turkey, 27 patients with a bile leak were randomized into 2 groups (sphincterotomy alone or stenting with or without sphincterotomy). They were allocated into subgroups according to the diameter of the common bile duct. The authors observed a leak closure rate of 84.6% in the sphincterotomy group versus 100% in the stenting group [18]. However, Rainio et al. [19] retrospectively analyzed 71 patients with a biliary leak who underwent sphincterotomy alone or sphincterotomy and stenting and found no significant difference in the primary healing rate between the sphincterotomy group and the sphincterotomy-plus-stenting group (90 vs. 90.4%). Mavrogiannis et al. [20] randomized 52 patients into 2 groups. The first group included 24 patients treated with a 7-Fr biliary stent alone, and the second group had 28 patients admitted for sphincterotomy followed by the insertion of a 10-Fr stent. The researchers observed a leak-closure rate of 100% in both groups, concluding that a small stent is as effective and safe as sphincterotomy and a large-bore stent. In a study from India, 72 patients were treated with sphincterotomy followed by nasobiliary drainage, plastic-stent placement, or nasobiliary drainage alone without sphincterotomy, and the success rate was 100% in all groups [21]. Recently, Haidar et al. [22] reviewed 100 cases of biliary leaks (14% were HG) treated with stent insertion alone, sphincterotomy alone, or combination therapy. In the subgroup analysis, the success rate of procedures with stent insertion (with or without sphincterotomy) was significantly higher than that of procedures without stent insertion (95.3 vs. 72.7%, p < 0.05). The failure rate of sphincterotomy-alone procedures (3/11, 27%) was significantly higher than that of procedures with stent insertion (4/85, 5%; p < 0.05). Kaffes et al. [23] analyzed 100 patients with a biliary leak who were treated with sphincterotomy alone, stenting alone, sphincterotomy, or balloon sphincteroplasty plus stenting. Endotherapy was unsuccessful in 7 patients (6 in the sphincterotomy-alone group; p = 0.001). Sandha et al. [2] conducted a retrospective review of 207 patients, and found that HG were better managed with a combination of stents and sphincterotomy, suggesting that this approach has the highest potential to seal a leak, particularly in cases with more complex or problematic leaks. Furthermore, they found 7 failures in patients treated with sphincterotomy alone, and these required stenting for leak closure. Three recent studies have supplied more data. Vlaemynck et al. [24] conducted a systematic review with a meta-analysis using 11 studies. Network meta-analysis was performed to compare sphincterotomy alone versus stenting alone versus combination treatment. Stenting was further stratified into leak-bridging and short-stenting. In their conclusion, the authors recommend sphincterotomy with stenting if the biliary leak can be bridged. If not, stenting alone with a short stent may be preferred to avoid sphincterotomy-related complications. Abbas et al. [4] conducted a nationwide review of 1,028 patients and observed that combination or stent monotherapy had lower failure rates than sphincterotomy monotherapy [4].
There are no definitive guidelines focusing on the endoscopic management of biliary leaks. From the literature, the idea emerges that stenting with or without sphincterotomy has better outcomes than sphincterotomy alone, and that, eventually, patients with a large probability of reintervention or complex/HG leaks will benefit from combination therapy.
Small-Diameter versus Large-Diameter Stents
Whereas stenting with or without sphincterotomy seems to be a key factor in the reduction of biliary system pressure after the diagnosis of a biliary leak, the optimal size of the stent introduced during endotherapy is still a matter of debate. Diameters <10 Fr was considered to be small and ≥10 Fr was considered a large diameter. There are only a few studies available for analysis in the literature. In the abovementioned study by Mavrogiannis et al. [20], there were no differences between patients treated with a 7-Fr plastic stent and those who underwent combination therapy using a 10-Fr stent. In another study from Greece, after performing sphincterotomy in all cases, patients were randomized to placement of either a 7-Fr or 10-Fr straight plastic stent for 4 weeks [25]; at the end of endotherapy, the success rate was similar in the 2 groups (93.54 vs. 96.87%, respectively). Other studies, although not designed to investigate this issue, presented similar results [2, 26]. In the abovementioned systematic review with meta-analysis, after a pooled analysis of 8 studies, the risk of failure after endotherapy was reported as the odds ratio (OR) [24]. The authors reported an OR of 0.47 (95% confidence interval [CI] 0.12-1.75) when comparing treatment with large-diameter (n = 136, success rate 97.8%) and small-diameter (n = 195, success rate = 95.4%) stents.
Complex and Refractory Leaks
Whereas the most frequent and more simple leaks (Strasberg Type A or Amsterdam type A) are very successfully treated with biliary sphincterotomy, placement of plastic stents, or both [1-3, 6], the optimal approach for more complex and refractory leaks has not been determined.
In 2006, Baron and Poterucha [6] first reported that 3 complex biliary leaks were treated successfully by using covered, expandable metal stents. They stated that the stents could stay in place for an arbitrary time of <3 months and did not have to be placed above the site of the leak to be effective, and that the successful closure of the leak was due to the diversion of bile away from the leak through the large-diameter stent. Three years later, a group from the USA reported a case series of 13 patients with complex leaks who underwent placement of fully covered, self-expandable metal stents (FCSEMSs) [27]. Although the researchers reported a success rate of 100%, there were several complications, and the prolonged time of stenting was associated with most of the adverse events. However, a study from Portugal evaluated 17 patients with refractory biliary leaks who underwent placement of an FCSEMS for ≤30 days [3]. The authors observed a 100% success rate without adverse events, suggesting that short-term stenting is efficacious and not associated with adverse events. Furthermore, in biliary ducts where the diameter at the site of the leak is <10 mm, the FCSEMS directly covers the leak site, increasing the potential for sealing the leak. However, in patients with a refractory biliary leak, instead of using an FCSEMS, the endoscopist can place >1 plastic stent which can theoretically manage the refractory bile leak at a lower cost. In a retrospective multicenter study, 178 patients with a biliary leak were treated with combination therapy (biliary sphincterotomy and placement of a 10-Fr plastic stent), which yielded a success rate of 91% [1]. The remaining 16 patients had a HG leak, and in this group, placement of multiple (≥3) large-bore (10 Fr) plastic stents resulted in clinical success in 10 patients (62.5%). The remaining 6 patients were successfully treated with placement of an FCSEMS, resulting in leak closure in all cases. After this suggestion of a better performance of FCSEMSs, only 1 study comparing the clinical success of FCSEMSs with the placement of multiple plastic stents (MPSs) became available [28]. After endotherapy, leak closure was accomplished in 13 patients (65%) who received MPSs, and in 20 (100%) who received FCSEMSs (p = 0.004). Furthermore, in the successful cases, the median time to leak closure was 11 days in the MPS group and 3.5 days in the FCSEMS group. The use of <3 plastic stents (p = 0.024), a summed plastic stent diameter below 20 Fr (p = 0.006), and a HG biliary leak (p = 0.015) were shown to be significant predictors of treatment failure with MPSs.
Taken together, these studies suggest that leaks that persist after combination therapy with sphincterotomy and a single plastic stent should be considered for treatment with an FCSEMS, especially in the face of a HG leak (Fig. 2).
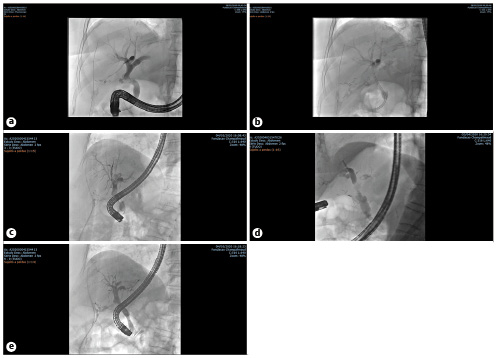
Fig. 2: Fluoroscopic images of endoscopic management of a refractory biliary leak using a fully covered self-expandable metallic stent (FCSEMS). a A high-grade (HG) biliary leak from cystic stump. b After endoscopic placement of a single plastic stent. c Persistence of a HG biliary leak after 5 days of plastic stenting. d Immediately after endoscopic placement of a FCSEMS with immediate reduction in the contrast flow through the leak. e Follow-up cholangiography immediately after FCSEMS removal at week 4. No contrast media extravasation is seen.
Stent Removal
Several studies report the time for leak closure using plastic stents. Most authors recommend the removal of plastic stents 4-8 weeks before stent obstruction, and this procedure is usually safe and without adverse events [1, 20, 25]. Ultimately, due to the rapid recovery of most patients, stents can be removed even earlier (i.e., before discharge from the hospital). Complications from the use of FCSEMSs in benign conditions have been reported [1, 3, 27]. These include stent migration, bile duct and duodenal ulceration, bile duct strictures, and de novo choledocholithiasis, with long-term stenting further increasing the complication rate. The optimal duration for which an FCSEMS should remain in place for the management of bile leaks is a matter of debate [3, 6, 27]. Nevertheless, there is some evidence that when stents stay in place for 3-4 weeks, leak closure and a low rate of adverse events can be achieved [1, 3, 28]. Stents can be safely removed by grasping with a snare or foreign-body forceps, and applying constant traction [1, 3, 28, 29].
Conclusions
Biliary sphincterotomy, in combination with the placement of a single plastic stent for short periods, is associated with leak closure in >90% of patients with postcholecystectomy bile leaks. Whereas there is no strong evidence that 10-Fr plastic stents are better than smaller ones, we concede that a larger stent may prevent clogging and obstruction, thereby assuring patency for longer periods. This option may be indicated when the leak flow is higher.
Refractory biliary leaks and ab initio HG or complex leaks should be treated with the placement of large-caliber MPSs (ideally, ≥3, summing to at least 20 Fr) or FCSEMSs, with an expected closure rate of >90%. All types of treatment with MPSs for refractory biliary leaks that are not successful should cross over to an FCSEMS that should stay in place for no longer than 3 or 4 weeks.
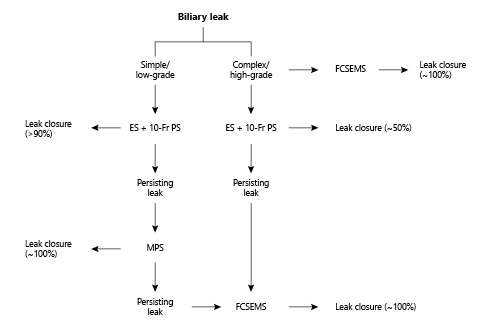
There are very limited indications for surgical biliary duct repair or percutaneous biliary drainage after ERCP in patients with postsurgical biliary leaks. An algorithm for the endoscopic management of biliary leaks is proposed in Figure 3.