Introduction
Clinical management of infected walled-off pancreatic necrosis (WOPN) has always been challenging. Currently, the standard treatment for WOPN is a step-up approach or surgical intervention with necrosectomy and drainage [1]. Conventional surgery is generally the only possible treatment option at most centers despite its high complication rates. Minimally invasive approaches such as minimally invasive retroperitoneal surgical necrosectomy, percutaneous catheter drainage, and endoscopic transluminal drainage are thought to induce less physiological stress owing to minimal activation of the inflammatory processes compared with conventional surgery [2-4]. Endoscopic drainage requires suitable anatomy for the collection to be accessible from a stable location in the gastrointestinal tract for adequate drainage. Occasionally, the collection can extend either caudally along the paracolic gutters or cranially to the lesser sac. WOPN extending into these locations is always challenging to manage, especially if a patient has multiple, non-communicating collections [5]. In this case report, we describe a novel approach for the treatment of a similar WOPN with ill-defined walls via percutaneous direct endoscopic necrosectomy (p-DEN) using an esophageal self-expanding metal stent (SEMS).
Case Report
A 59-year-old female with hypertension and hypothyroidism on medications was recently diagnosed with severe acute necrotizing pancreatitis with an acute necrotic collection (Fig. 1a) which was managed conservatively in September 2018. A month later, the patient presented with fever, abdominal pain, and hypotension. On examination, the patient had diffuse tenderness over the abdomen. The patient was resuscitated with parenteral fluid therapy, non-invasive ventilation, and vasopressors. Empirical parenteral antibiotics (cefoperazone 1,000 mg + sulbactam 500 mg IV Q12H) were started after collecting blood cultures. Laboratory tests demonstrated hemoglobin of 8.6 gm/dL, leukocytosis with a left shift, high C-reactive protein of 120 mg/L, and a PaO2/FiO2 ratio of 234. Liver and renal function tests were within the normal limits. The patient underwent a computed tomography (CT) scan of the abdomen which showed a large ill-defined necrotizing peripancreatic fluid collection containing air foci. This collection replaced the neck, body, and tail, extending superiorly to gastrosplenic fat and bilaterally along the paracolic gutters. There was no evidence of any bowel leak (Fig. 1b, c).
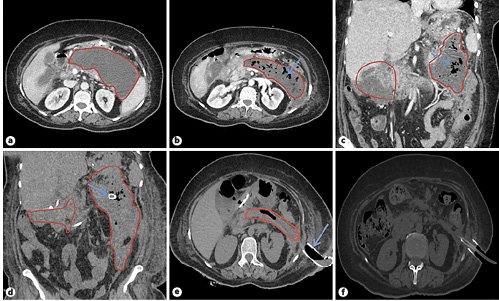
Fig. 1: a Baseline CT abdomen depicting severe acute necrotizing pancreatitis with an acute necrotic collection. b, c CT abdomen after 4 weeks showing a large necrotizing peripancreatic fluid collection containing air foci (arrow), replacing the neck, body, and tail of the pancreas (measuring 14 × 4 cm in the transverse axis). There is continuity to the retrogastric region extending superiorly to gastrosplenic fat, to the right side along the hepatic flexure of the colon and in the subhepatic-pericholecystic region (measuring 8.6 × 7.8 × 4.8 cm), to the left side along the left anterior pararenal fascia medial to the left colon, and extending posteriorly in close association with the left psoas muscle (measuring 5.2 × 5.3 × 2.3 cm). d CT-guided percutaneous drainage using a 12-Fr percutaneous pigtail catheter seen in situ (arrow). e CT showing a percutaneously placed SEMS for performing p-DEN via a retroperitoneal route. f CT after 4 sessions of p-DEN showing resolution of the WOPN.
Despite being on antibiotics, the patient did not improve clinically even after 24 h and was planned for a CT-guided percutaneous drainage. Following a step-up approach, a 12-Fr percutaneous pigtail catheter was inserted into the WOPN from the left subcostal site, and fluid aspirate was sent for culture which grew multidrug-resistant Klebsiellapneumoniae. Antibiotics were tailored as per the culture sensitivity pattern (meropenem 1 g IV loading dose, maintenance 1 g/Q8H and colistin 9-MIU IV loading dose, maintenance 4.5-MIU/Q12H) and continued up to 18 days (Fig. 1d). However, the patient condition continued to worsen despite upgrading the size of the pigtail catheter to 26 Fr (anticipating better drainage). Therefore, it was decided to use a percutaneous access to enter the WOPN for thorough debridement and washout using flexible endoscopy by placing a wide bore fully covered esophageal SEMS.
Under propofol-based total intravenous anesthesia (TIVA), we replaced the pigtail catheter by an esophageal SEMS using the Seldinger technique. The WOPN was visualized by contrast injection through the pigtail catheter under fluoroscopy. A 0.035-inch guidewire (Dreamwire, Boston Scientific, USA) was introduced through the pigtail catheter, and the latter was removed, with the wire was left in place. Subsequently, a 20-mm dilation balloon (CRE Wireguided, Boston Scientific) was used to dilate the tract over the guidewire under fluoroscopic guidance to facilitate SEMS placement. A WallFlex fully covered esophageal stent (Boston Scientific) with a diameter of 20 mm and a length of 100 mm was inserted over the guidewire into the cavity. A flexible gastroscope (GIF-1TQ190, Olympus, Japan) was introduced through the SEMS and DEN was performed through the stent by irrigation with sterile saline, diluted betadine and hydrogen peroxide, and a combination of stone retrieval basket and polypectomy snares. To provide continuous drainage, we inserted a 7-Fr pigtail stent (plastic biliary stent, Boston Scientific) through the SEMS. The SEMS and the plastic stent were secured by suturing them to the skin (Fig. 1e).
We repeated the necrosectomy procedure (in total 4 sessions over a period of 2 weeks, under TIVA) until collection resolution (Fig. 1f). We covered the tract with a colostomy bag and changed it as per requirement. The deployment of percutaneous SEMS and p-DEN are depicted in Figure 2a-f. The patient showed considerable clinical improvement and was discharged 3 days after the last necrosectomy session. The patient was followed up in the outpatient clinic after 2 weeks. The drainage output progressively declined and after 4 weeks of SEMS placement it was removed and the track closed by secondary intention within 3 months.
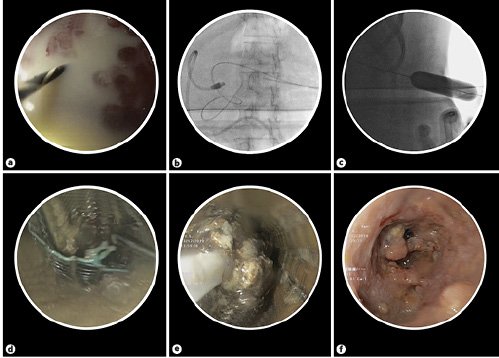
Fig. 2: Technique of percutaneous SEMS placement. a Removal of the small caliber plastic pigtail catheter (on the left side) over the guidewire. b Fluoroscopic image showing the guidewire and the right sided pigtail catheter. c Wire guided CRE balloon dilatation to facilitate SEMS placement. d Esophageal SEMS deployed percutaneously along the dilated tract for p-DEN. e Necrotic material being removed using a stone retrieval basket. f Endoscopic view of the WOPN cleared of infected debris following p-DEN.
Discussion
By using an esophageal SEMS and performing a percutaneous retroperitoneal approach, we successfully performed p-DEN of the abovementioned ill-defined WOPN. This is the first of such cases in an Indian context whereby an esophageal SEMS is used for p-DEN. Currently, many endoscopic drainage options exist to improve the step-up approach for WOPN in order to avoid surgical necrosectomy [6]. A literature search resulted in 9 studies describing p-DEN using a percutaneous SEMS in the treatment of necrotizing pancreatitis. This included 2 case series and 7 case descriptions [7-15]. Thorsen et al. [13] reported 5 cases with a technical success of 100%, while the clinical success was 80% after an average of 5.75 necrosectomy sessions. Tringali et al. [14] reported 3 cases of WOPN resolution by percutaneous necrosectomy through SEMS after an average of 3 endoscopic sessions. No procedure-related adverse events were seen. Saumoy et al. [15] reported 9 patients with WOPN who underwent p-DEN which was combined with endoscopic transmural drainage and necrosectomy (ETDN) with 100% technical success and 89% clinical success rates.
This patient had a large collection (WOPN) with paracolic extension which was not accessible endoscopically. The abdominal CT scan suggested that it could be approached through a retroperitoneal route. Thereafter, an initial step-up approach with plastic stents was not clinically effective. Hence, a decision was taken to drain the WOPN using a minimally invasive approach (P-DEN) as the patient was not fit for major surgery. We accessed the WOPN via a retroperitoneal approach rather than a transperitoneal approach as the former has fewer complications [16]. Also, we relied solely on p-DEN rather than a combination of p-DEN and ETDN owing to the ill-defined walls in our case. The WOPN walls were not mature even after 4 weeks and we were compelled to make an early decision for p-DEN. Corroborating our approach, a recent study showed better outcomes with less organ failure and in-hospital mortality when percutaneous drainage is performed early in the course of necrotizing pancreatitis [17]. There have been reports of drainage through single or multiport percutaneous routes and through sinus tract endoscopy [17, 18]. However, we felt that using a SEMS might help in increasing the technical success. Larger collections can also be drained with minimal invasive techniques. MRI is preferred over CT to assess drainage because it is better at detecting non-liquefied material [19].
An RCT [1] showed the superiority of a step-up approach using video-assisted retroperitoneal debridement (VARD) over open surgery, whereas another RCT showed a superiority of ETDN over surgery [4]. A recent RCT showed the advantages of an endoscopic step-up approach compared to a step-up approach of percutaneous catheter drainage and VARD [20]. EUS-guided transmural drainage has also shown similar technical and clinical success compared to percutaneous approaches [21]. Owing to multiple results seen from these trials, there is a need for randomized studies with large sample sizes followed by appropriate systematic reviews. The ideal drainage for this collection would have been a dual approach of EUS cystogastrostomy and a percutaneous technique. Since this was a very large collection with paracolic extension, we decided to initially proceed with the percutaneous approach [22] followed by EUS drainage if needed. We placed a SEMS and through the SEMS we could perform DEN. We achieved a good necrosectomy response over a few sessions using this approach as the symptoms gradually reduced and the size of collection decreased. In view of improving clinical and laboratory parameters, we continued to perform DEN through this route and achieved a near complete necrosectomy response, thereby avoiding an additional EUS drainage and its added costs.
Navarrete et al. [3] proposed percutaneous access via a large-bore esophageal SEMS with the aim of making access to the necrotic cavity easier and faster. The primary indication for p-DEN is the presence of infected necrosis. If the WOPN becomes obstructed or fistulized to adjacent anatomical structures, compressing into the surrounding vasculature, the patient needs rapid intervention. This technique has numerous advantages. It allows for a wide opening access and better stability of the scope (as compared to enteral and colonic stents) for endoscopic intervention with standard or even therapeutic endoscopes. A benefit of using SEMS in place of a large bore catheter is to sustain the patency of the tract and does not require re-dilatation during subsequent sessions, thereby allowing a permanent wide duct for drainage. Furthermore, it provides a larger drainage lumen which prevents obstruction from thick, tenacious necrotic material, which is common with WOPN. It allows easier observation of the drainage site and output between necrosectomy sessions. The collections can be drained on the most declivous side which guarantees better emptying in comparison to a transgastric approach, even when it would be feasible. Finally, p-DEN through SEMS can be performed with standard or therapeutic endoscopes under TIVA without the need for general anesthesia, which is often required for prolonged per-oral endoscopies. Due to its promising results as seen from multiple cases, p-DEN can be used in the step-up approach for the management of WOPN. Disadvantages include more pain, stent dislocation, and fistula formation. Also, the question of possible infection access through a large bore stent is a matter of concern. Repeated procedures are often needed and may not be useful in the case of extensive necrosectomy [13]. This procedure can only be performed by experienced interventional endoscopists with optimal radiological and surgical facilities in a tertiary care center [14].
To summarize, wide retroperitoneal access to WOPNs with SEMS followed by endoscopic necrosectomy is a safe and effective intervention in appropriately selected patients. These results need to be reproduced with a large patient cohort before this becomes the standard of care in this patient population.














