Introduction
Anorectal pain is a symptom with a negative impact on quality of life and can sometimes develop into a chronic pain syndrome [1]. The aetiology can generally be divided into 2 types: (a) structural disorders such as anal fissures, fistulae, haemorrhoids, and disorders often associated with infection, thrombosis, or necrosis which can subsequently lead to pain; and (b) functional anorectal disorders, such as levator ani syndrome, proctalgia fugax, and unspecified anorectal pain [2, 3]. Structural disorders are treated according to their underlying pathology and surgery is an option in many cases. Functional disorders are treated initially with conservative measures such as lifestyle changes, diet, laxatives, and pelvic floor musculature physiotherapy. When these measures fail, both functional and structural disorders can be challenging to treat [4].
While anorectal surgery is generally well tolerated, short- and long-term complications frequently occur. Acute complications include bleeding, infection, and urinary retention. Long-term complications include faecal incontinence (FI), anal stenosis, and chronic pelvic pain (CPP) [5]. In chronic post-surgical anorectal pain (CPAP) that is refractory to conventional therapeutic approaches, percutaneous tibial nerve stimulation (PTNS) can be an option [6].
Previous literature shows that neuromodulation can be an alternative in cases of chronic anal fissure (CAF). Studies show that sacral nerve stimulation (SNS) is an effective alternative therapeutic option for CAF in patients who choose not to pursue more invasive surgical interventions [7]. The effect of SNS on anorectal function occurs at the pelvic afferent or central level via the S2-S4 nerves which contain fibres of the pudendal nerve and afferent sensory fibres from the anal sphincter and pelvic floor [8]. Based on this, Altunrende et al. [9] investigated the effect of a less invasive treatment with transcutaneous tibial nerve stimulation (TTNS) in patients with CAF. They concluded that TTNS application to the posterior tibial nerve has the potential to be an alternative treatment option for patients who seek a non-invasive treatment modality.
PTNS is another treatment option for stimulation of the tibial nerve. It is postulated that it exerts its effect through indirect modulation of the sacral plexus via the posterior tibial nerve which contains sensory, motor, and autonomic fibres from the fourth lumbar to third sacral roots. Its mechanisms of action are yet to be fully understood, but extrapolation from SNS evidence suggests sensory and motor neuromodulatory effects [8].
SNS is an invasive technique that has been used for urinary symptoms and FI, and it has been more extensively studied than PTNS. However, it requires the surgical placement of electrodes, so interest in less invasive techniques has grown [10]. The tibial nerve is easily accessible and provides an optimal site for neurostimulation [11]. The most common complications of needle placement are minor: bleeding, pain, localised muscle-cramping, generalised swelling, and, occasionally, occipital headaches [12].
PTNS is a neurostimulation technique commonly used in the treatment of urinary urgency, frequency, and retention as well as urge incontinence [11]. There has been increasing evidence of its benefits in improving other conditions such as CPP [6, 11] and FI [13]. In this context, previous clinical trials and cases series achieved a reduction in the number of incontinence episodes and an increase in the deferral time for deposition. Improvements in FI severity and quality of life scales were also achieved [13].
Case Report
A 45-year-old woman, whose past medical history included 5 surgeries for complex anal fistulae, was referred to our institution’s Chronic Pain Unit (CPU) by her general practitioner with a 4-year history of post-surgical anorectal pain. The surgeries were described as complex procedures, with one involving exploration of the sphincter with anatomical correction.
Pain medication history included gabapentinoids, tramadol, oxycodone, and amitriptyline, with minimal pain relief. Other causes of anorectal pain had been previously ruled out by thorough testing, namely endoanal ultrasound, video-defecography, and manometry.
At the first visit, she reported pain symptoms in the endoanal area but no complaints in the external perianal region. She described a persistent, burning, and “screw-like” pain. Pain was associated with dyschezia and FI, with leakage of solids and gas. There was marked interference of her sleep and a negative impact on her quality of life. No ameliorating factors were reported. The patient’s Numeric Pain Scale (NPS) scores were 6/10 at the first visit and 6/10 as the worst pain in the previous 7 days.
In May 2015, PTNS became available in the CPU and the patient was enrolled for this treatment. The treatment protocol consisted of 12 once-weekly 30-min sessions. Treatment effectiveness was monitored at the beginning of every session and at the end of treatment (Table 1). A 34-gauge needle was placed 1 cm posterior and 3 cm proximal to the medial malleolus. The Urgent PC© neuromodulation system (Cogentix Medical, Inc., Minnetonka, MN, USA) was used for stimulation (Fig. 1). The frequency was 20 Hz and the pulse width was 200 μs. The current was gradually increased until a motor response was observed (flexion of the toes or plantar flexion of the foot) to determine the appropriate stimulation amplitude (possible between 0 and 9 mA) and confirm correct needle placement. After this, the 30-min timed therapy session was started (Fig. 1).
Table 1 Evaluation of pain characteristics and functional interference evolution throughout treatment
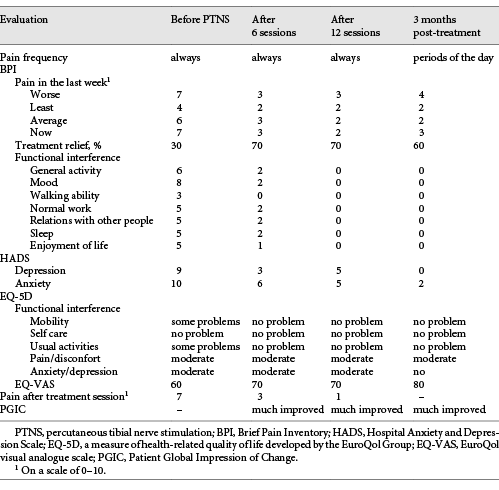

Fig. 1 Illustration of the percutaneous tibial nerve stimulation (PTNS) technique. The patient is in a sitting position with a support for her foot, and the foot is positioned in lateral rotation. The needle is placed 1 cm posterior and 3 cm proximal to the medial malleolus, attached to the Urgent PC© neuromodulation system (Cogentix Medical, Inc., Minnetonka, MN, USA).
During treatment, gabapentin 100 mg twice daily and oxycodone/naloxone 5/2.5 mg and amitriptyline 5 mg once daily was maintained. No contraindications for the procedure were identified.
The impact of PTNS on the patient’s pain and daily activities was evaluated with scales that are in common use in our practice: the Brief Pain Inventory (BPI), the Hospital Anxiety and Depression Scale (HADS), the EuroQuol Group Questionnaire (EQ-5D-3L), and the Patient Global Impression of Change (PGIC). Results are shown in Table 1.
By the end of the sixth PTNS session, the patient’s symptoms had substantially improved. She reported a progression from 30 to 70% pain relief with the treatment. Pain intensity was significantly reduced, as well as indices of anxiety and depression, as assessed by HADS (Table 1). With the continuation of treatment, the pain scores consistently decreased. By the end of the 12 sessions, the patient had a substantial decrease in both the frequency and intensity of pain. A decrease in the interference of pain with mood, normal work, and walking/mobility was also noted, as assessed by the BPI and EQ-5D-3L questionnaires (Table 1). Although not systematically evaluated, the patient reported an important benefit in FI, with no episodes occurring after conclusion of the 12 sessions.
Substantial and sustained improvement in parameters related to quality of life and pain intensity were maintained at treatment follow-up 3 months after the last session. The patient experienced a significant reduction in the interference of pain with mood, walking, and normal work throughout the treatment period and 3 months after treatment. She also reported a global benefit to her health and an improvement in her quality of life.
Follow-up has continued until the time of writing of this paper. No relapse of symptoms occurred and no further PTNS sessions were required. The reduction in pain intensity allowed the suspension of amitriptyline from the patient’s usual pain medication, while permitting superior pain control with the same doses of gabapentin and oxicodone/naloxone.
Discussion
Although chronic anorectal pain is relatively common, few studies have focused on this problem. It is defined as recurrent or persistent pain in the anal canal, usually deep and severe, that lasts for > 3 months. Patients generally present with a combination of symptoms that can include pain, soiling, loss of blood or pus from the anus, FI, and a palpable mass in and around the anus. The combination of symptoms suggests the presence of disease, and a clinical examination is performed to assess local anal abnormalities, including anal fistula, complicated haemorrhoids, hypertrophied anal papillae, anal fissure, and perianal suppurative conditions [14].
When the only presenting complaint is pain in the anal region and any previous local abnormality has been treated, with investigations unable to identify the cause of pain, the clinician is faced with a challenge. In this case, after a complete investigation to exclude other causes of pain, the diagnosis of CPAP is assumed. This is chronic pain that develops or increases in intensity after a surgical procedure and persists beyond 3 months [15].
Neuromodulation
Neuromodulation has been studied for decades, but its mechanism of action is not yet completely understood. Many theories exist. (a) In lower urinary tract dysfunction, it is thought that neuromodulation causes rebalancing of inhibitory and excitatory impulses in the central nervous system, that are responsible for bladder function [16]. (b) Regarding the impact of neuromodulation on pain, gate control theory suggests that stimulation of large somatic fibres modulates thinner afferent AC or C fibres, decreasing pain perception [17]. (c) Transcutaneous electrical and acupuncture therapy nerve stimulation lead to elevation of the endorphin levels at the spinal level [18, 19].
Percutaneous Tibial Nerve Stimulation
The tibial nerve is a sensory and motor nerve that originates from spinal roots L4-S3, which also contribute directly to sensory and motor control of the urinary bladder and pelvic floor. The interest in the tibial nerve pertains to its ease of access via surface landmarks [11].
PTNS is typically performed with patients in the supine position, with the knees abducted and the soles of the feet together. A 34-gauge needle is inserted into the skin 3 fingerbreadths cephalad to the medial malleolus. A grounding pad is placed on the arch of the ipsilateral foot. The amplitude of the stimulation is increased until the first toe curls or the toes fan. This technique has the advantage of being performed without requiring an operating room or anaesthesia [11].
PTNS has been more extensively studied in the treatment of pelvic organ dysfunctions commonly associated with CPP. Recent reviews show promising results both in lower urinary tract dysfunction [11], FI [13], and as a treatment option for CAF [8]. However, larger-scale studies are needed and there is no published evidence of PTNS efficacy on chronic anorectal pain.
There are no recommendations available for the ideal PTNS regimen. We therefore applied the most commonly described regimen, which consists of 30-min sessions repeated once a week for 12 weeks. Both motor and sensory responses have been described as good endpoints to confirm correct needle placement, but in this case a motor response was achieved in every treatment session. After initial treatment, PTNS can be performed as a long-term treatment to maintain the achieved benefits [20].
Larger-scale studies are needed to assess the clinical efficacy of this approach, as a single case is not sufficient to determine the important issue of the relative proportion of the placebo effect.
Conclusion
PTNS may be effective in refractory cases of CPAP, similarly to its effect on functional anorectal chronic pain syndromes, previously described in the literature. It is a novel, minimally invasive, neurostimulation modality with a low rate of complications that can be performed on an outpatient basis.
The overall improvement observed in this specific case of CPAP suggests a new area of research for PTNS. Further investigation is needed to establish PTNS as a possible alternative treatment modality for this condition.














