Introduction
There is an increased risk of intestinal and extra-intestinal cancers in patients with inflammatory bowel disease (IBD) [1, 2]. Furthermore, as the population with IBD ages, it is not uncommon to encounter patients with a history of cancer, which poses therapeutic challenges, requiring multidisciplinary decisions. IBD treatment in the setting of prior cancer is complex and must consider several aspects such as the type of cancer and its potential for recurrence, as well as the severity of IBD and therapeutic alternatives. Most IBD drugs produce some level of immunosuppression, and therefore there is a concern that their use in patients with a prior history of cancer may increase the risk of new or recurrent cancer. There is evidence that patients with IBD treated with thiopurines exhibit an increased risk of lymphomas and nonmelanocytic skin cancers while those exposed to anti-TNF-α agents are at an increased risk of melanoma [3]. However, registry data and mostly observational retrospective studies across immune-mediated diseases have shown that the use of biologics in the setting of a prior cancer does not seem to increase the risk of incident or recurrent cancer. In a retrospective cohort study, looking at IBD patients with a history of cancer, exposure to anti-TNF-α agents alone or in combination with thiopurines was not associated with a risk of new or recurrent cancer in the 5-year follow-up period, even after adjusting for cancer recurrence risk [4]. The data available with newer biologics, such as vedolizumab (VDZ) and ustekinumab, are still limited, but overall, no major safety signal has yet emerged [5]. Herein we present the case of a 76-year-old man with a history of melanoma and steroid-dependent left-sided ulcerative colitis (UC), refractory to mesalamine and thiopurines, who went into clinical and endoscopic remission after starting VDZ and who was diagnosed with a multifocal colorectal cancer (CRC), shortly after. We discuss the challenges of treating IBD patients with a history of cancer and the potential role of VDZ in this setting.
Case Report
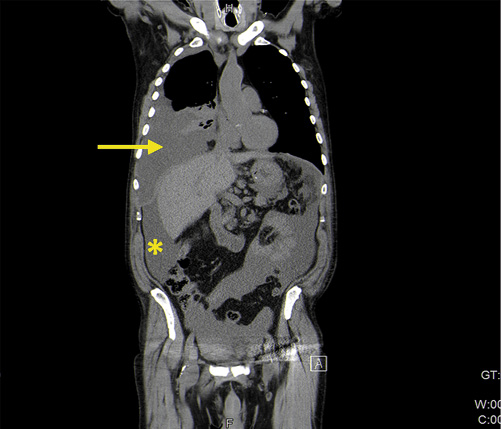
We present the case of a 76-year-old man, former smoker (40 pack-year units) with a medical history of arterial hypertension, psoriasis since the age of 23 treated with topical corticosteroids and calcitriol, and a prostatectomy due to benign prostate hyperplasia. He had no familial history of IBD or CRC. In 2009, he developed diarrhea and bloody stools and was diagnosed with left-sided UC. He was started on oral and topical mesalamine with good response. In 2011, a right scapula malignant melanoma stage Clark III was surgically removed. The surgery was curative, and the patient had no indication for systemic therapy, so he maintained the follow-up at a dermatology consultation. In 2013, he was referred to our IBD outpatient clinic. He complained of rectal bleeding, with around 6 bowel movements per day of liquid stools and fecal urgency, sometimes with incontinence. Laboratory workup showed hemoglobin of 13.4 g/dL, platelets of 320 × 109/L and C-reactive protein of 1.26 mg/dL. Colonoscopy revealed mucosal hyperemia, superficial ulcerations and pseudopolyps in the rectum and sigmoid colon (Mayo 3) (Fig. 1). Colonic biopsies were consistent with active UC without dysplasia or cytomegalovirus infection. Oral and topical mesalamine were dose-optimized, with partial response only, requiring the addition of topical and oral steroids. The clinical course was marked by frequent relapses that required oral steroids. For this reason, a need for therapy escalation was discussed with the patient and with the dermato-oncologist. Given the recent history of melanoma, Dermatology considered that there was a relative contra-indication to start anti-TNF-α and the patient was started on azathioprine in 2016. Surgery was also discussed, but not considered an option by the patient. While on azathioprine, he had soft stool with a frequency of 3-4 bowel movements per day without blood. However, fecal urgency persisted and on endoscopy erythema, loss of vascular pattern and spontaneous bleeding were noticed (Fig. 2). In addition, azathioprine was stopped in 2017 due to a severe flare of genital herpes. The patient remained symptomatic, and endoscopy showed pseudopolyps, a tubular-looking colon with superficial ulcers in the sigmoid colon and rectum (Mayo 3). During this period, he was maintained on topical mesalamine. Considering the patient’s advanced age and prior history of melanoma, he was started on VDZ in January 2018. Clinical remission with a stool frequency <3/day with no bleeding was achieved after 8 months of VDZ. The rectosigmoidoscopy showed endoscopic healing in the sigmoid colon and rectum, with a few colonic pseudopolyps (Fig. 3). Laboratory evaluation showed no anemia (hemoglobin 13.6 g/dL) or thrombocytosis, C-reactive protein of 1.07 mg/dL and albumin of 4.4 g/dL. In February 2019, nine years after the initial diagnosis, the patient underwent his first surveillance colonoscopy. In the sigmoid colon, with the mucosa healed, it was possible to visualize two polyps of 5 and 10 mm (Paris 0-IIa) with adenomatous appearance in the descending and sigmoid colon. Pathology showed tubular and serrated adenomas with low-grade dysplasia on histological examination. Simultaneously, three different areas of flat mucosa in the rectum and sigmoid with an irregular crypt pattern were identified (Fig. 4). Biopsies of these areas revealed a synchronous poorly differentiated adenocarcinoma (Fig. 5). Carcinoembryonic antigen was 15.5 U/mL. No loss of DNA mismatch repair proteins was detected (low probability of microsatellite instability), the KRAS, NRAS or BRAF mutations were negative, and the mutation for the human epidermal growth factor receptor 2 (HER2) was positive. Soon after the diagnosis of colorectal cancer he presented with abdominal distention and shortness of breath. Computed tomography of the chest-abdomen and pelvis showed a large-volume pleural effusion, ascites and a large epiploon densification (Fig. 6), consistent with secondary dissemination. Both ascites and pleural fluid cytology were positive for malignant cells. No liver or lung metastases were detected. After discussion in a multidisciplinary meeting, given the diagnosis of stage IVB colon adenocarcinoma with peritoneal and pleural involvement, dexamethasone and palliative systemic chemotherapy (cetuximab and FOLFIRI) were initiated. The patient’s condition rapidly declined, and he died 3 months later. A notification of this case was reported to the INFARMED’s national pharmacosurveillance program.
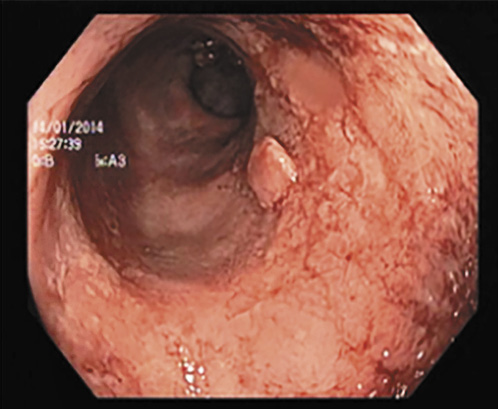
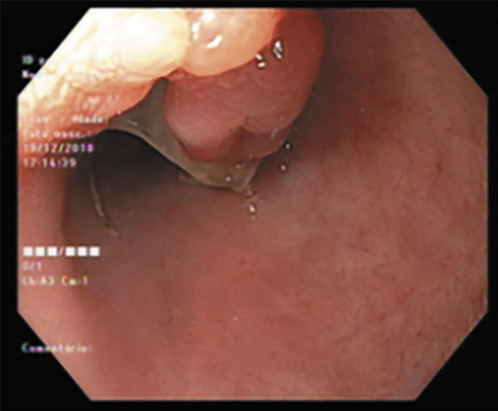
Fig. 1 Colonoscopy showing rectal involvement with mucosal friability, superficial ulcerations and pseudopolyps (Mayo 3) in 2013.
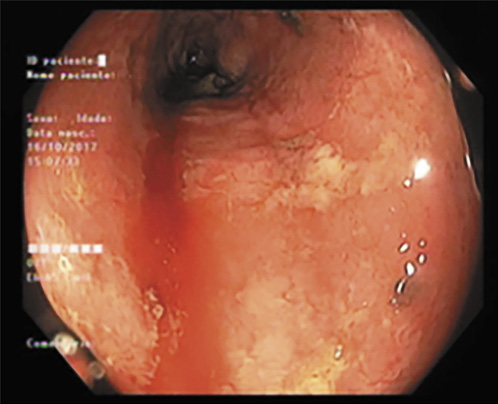
Fig. 2 Rectosigmoidoscopy revealing marked erythema, loss of vascular pattern and spontaneous bleeding (Mayo 3) under treatment with azathioprine in 2017.
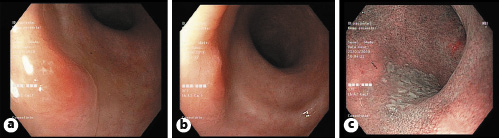
Fig. 4 Surveillance colonoscopy showing two areas of mucosal hyperemia with the use of light colonoscopy at 30 cm (a) and at 25 cm (b) of the anal margin and another whitish appearance mucosal area visible with narrow band imaging (c), all of them with malignant cells on microscopic examination.
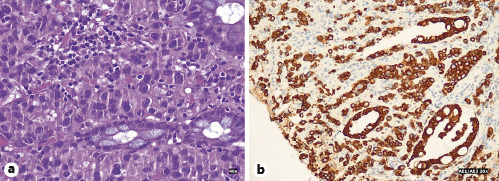
Fig. 5 Microscopic examination of biopsy specimen. a Poorly differentiated adenocarcinoma with inflammatory infiltrate and crypt distortion. Hematoxylin-eosin. ×40. b Immunohistochemical study with staining of cancer cells. AE1/AE3. ×20.
Discussion
The present report documents the clinical and endoscopic evolution of a patient with a left-sided UC refractory to mesalamine and thiopurines. The selection and escalation of treatment was particularly challenging given the patient’s advanced age and history of skin cancer. We also highlight the rapid progression and the unusual metastasization pattern of an aggressive CRC developed 1 year after VDZ introduction.
Patients with UC have an increased lifetime incidence of CRC compared with the general population. In a meta-analysis, Eaden et al. [6] estimated that the cumulative probability of cancer is 2% at 10 years, 8% at 20 years and 18% at 30 years from symptom onset. There are some factors that predict the risk of CRC, namely the duration, extension and inflammatory activity of the disease [7]. Our patient had a UC with 9 years’ duration with limited extension, albeit poorly controlled, which confers a low to intermediate risk of CRC. On the other hand, the persistent inflammatory activity (partial Mayo score >3 and endoscopic Mayo >2) until VDZ was started may have contributed to an increased risk of cancer. In this patient, therapy escalation and disease control were hindered by the history of melanoma. There is growing evidence linking anti-TNF-α therapy and an increased incidence of melanoma, due to changes in immune system checkpoints [3, 8]. In a study using the British Rheumatology Biologic Registry, the risk of recurrent melanoma was higher in the anti-TNF-α-exposed patient [9]. Altogether, these data, allied with the aggressiveness of melanoma, suggest caution in the use of anti-TNF-α in the context of this cancer and led our dermato-oncologist to suggest against this therapeutic class in our patient. On the other side, thiopurines have been linked to the nonmelanoma skin cancer and a higher risk of viral infections [3, 8]. Despite discussing with the patient the possibility of surgery as an alternative therapeutic option, this was refused.
Surveillance colonoscopy has been endorsed to detect early dysplasia and re-evaluate disease extension and activity. The American Society of Gastrointestinal Endoscopy and the British Society of Gastroenterology guidelines advocate screening colonoscopy beginning 8-10 years from disease onset [10, 11]. Although CRC is rarely encountered when disease duration is less than 8-10 years, a substantial part of IBD-associated CRC may occur before colonic surveillance. Lutgens et al. [12] identified 149 patients with IBD-associated CRC and showed that about one fifth of the patients developed cancer before colonic surveillance should start. This fact suggests that besides extent and disease duration, some other risk factors such as age at UC onset and family history of CRC may decrease the time span between UC diagnosis and CRC occurrence. Our patient underwent surveillance colonoscopy, while in remission, about 9 years after symptom onset. Colonoscopy revealed three areas where very subtle mucosa alterations, characterized by changes in mucosal pattern, were more easily identified with narrow banding image. As he had never been in endoscopic remission before, it could had been difficult to identify these areas on colonoscopy due to mucosal chronic inflammation or absence of malignancy at this time. Besides, flat dysplasia is difficult to delineate and high-definition endoscopes are necessary to distinguish it from inflammation. There are controversial data regarding dysplasia and cancer detection during surveillance colonoscopy in UC [13, 14]. Some studies demonstrated that surface pattern, as determined by narrow banding image magnifying colonoscopy, is useful for differentiation between UC-associated cancer/dysplasia and nonneoplastic lesions [14]. For this reason, the European Society of Gastrointestinal Endoscopy recommends the routine use of 0.1% methylene blue or 0.1-0.5% indigo carmine pancolonic chromoendoscopy with targeted biopsies and taking biopsies from flat mucosa surrounding neoplastic lesions or all suspicious lesions identified at neoplasia surveillance in long-standing colitis [15]. Flat dysplasia is rare but is more likely to be multifocal and often to progress to synchronous CRC compared with polypoid lesions as observed in our clinical case [10]. Besides the short time span between UC diagnosis and cancer development, with advanced stage of the CRC at diagnosis, what was also striking was the unusual spreading pattern of metastasis presented, since classic adenocarcinomas have a lower risk of peritoneal metastasis compared with mucinous and signet ring adenocarcinomas (20.1 vs. 48.2 and 51.2%) [16]. HER2 overexpression is a relevant genetic alteration occurring in 5% of patients with metastatic CRC, being a negative predictor of response to cetuximab [17]. This may explain the rapid disease progression under treatment. However, the prognostic role and influence in overall survival of HER2 in CRC remains uncertain [17]. Likewise, the potential role of VDZ in the atypical presentation of this CRC is uncertain, but merits reflection. VDZ is a humanized monoclonal antibody targeting α4β7-integrins approved for patients with moderate-to-severe IBD. Its safety profile has already been demonstrated in some clinical trials. Colombel et al. [18] gathered safety data from six clinical trials of VDZ where malignancy was reported in 18 of 2,830 patients exposed to VDZ. Six out of 18 of these patients had gastrointestinal malignancies, with 3 of them having CRC. These data are consistent with the expected risk observed in IBD patients [18]. In another systematic review and meta-analysis of randomized controlled trials from 49 studies reporting the incidence of malignancies, there was no association between VDZ exposure and cancer risk. However, this study is limited by short periods of exposure and follow-up [5]. A large retrospective multicenter cohort study concluded that the noninfectious serious events were low and were mostly related with the use of concomitant immunosuppressive therapy [19].
Although gut selectivity of VDZ may result in a more favorable safety profile in a high-risk population for cancer or infections, there is a theoretical concern, never confirmed, that by reducing the migration of activated leukocytes to the gastrointestinal tract, it may also reduce cancer immunosurveillance. A study with 68 patients treated with VDZ for more than 1 year found that ongoing VDZ responders with UC demonstrate mucosal healing after long-term follow-up. They also analyzed the colonic dysplasia and adenocarcinoma, identifying dysplasia in 10% of patients, but none of the other patients progressed to high-grade dysplasia or cancer [20]. In a retrospective observational study of 75 patients with IBD and primary sclerosing cholangitis, treated with VDZ for at least 30 weeks, 9 developed digestive cancer (7 CRC and 2 cholangiocarcinoma). Three cases of CRC were diagnosed between weeks 30 and 54 of therapy, and 4 cases were found between 1 and 3 years after the introduction of VDZ [21]. The development of digestive neoplasia might be attributable to the well-known increased risk of colorectal malignancy in this population, but the role of impaired immunosurveillance induced by VDZ should be evaluated. It can be postulated that the malignancy risk may be related to the impairment of the immunosurveillance of the gastrointestinal tract induced by anti-integrin antibodies [20]. More studies are necessary to address its effect on immunovigilance and the long-term malignancy risk. Finally, it is generally advised, solely based on the mechanisms of action, that this drug should probably be avoided in the setting of prior gastrointestinal cancers [22].
In summary, we present a clinical case of multifocal CRC detected 9 years after diagnosis, developing 1 year after VDZ therapy in a patient with poorly controlled left-sided UC. Strikingly, after achieving clinical and endoscopic remission, and 12 months after starting VDZ, the patient was diagnosed with CRC. The short time span between UC diagnosis and CRC detection in a patient with left-sided colitis, with multifocal poorly differentiated adenocarcinoma at diagnosis, and the atypical pattern of metastasization are unusual. Thus, we emphasize that comorbidities have important therapeutic implications in UC management and treatment should be individualized.