Introduction
Hemorrhoidal disease (HD) is very common among adults and is defined as the symptomatic enlargement and distal displacement of the normal vascular structures in the anal canal [1].
Treatment options depend on the type and severity of the disease, patients’ preferences, and physician’s expertise. There are several approaches such as lifestyle and diet modification, medical treatment (systemic and topical drugs), office-based procedures, and surgical treatments [2]. Internal HD grades I to III are usually treated with medical treatment and/or office-based procedures, with surgery being reserved for grade IV hemorrhoidal disease, external hemorrhoids, and disease refractory to office-based treatment [3].
The most used office-based procedures are rubber band ligation (RBL), sclerotherapy, and infrared coagulation [3]. However, few studies have been published comparing the various types of instrumental therapy. The latest meta-analysis comparing various hemorrhoidal therapeutic modalities was published 26 years ago (in 1995) [4]. In this meta-analysis the authors concluded that, among office-based therapies, RBL was the most effective, although more painful and more prone to bleeding. Since this publication, RBL is seen as the gold-standard office-based procedure and is recommended as the first-line treatment for hemorrhoidal disease grades I to III [4]. Since then, other alternatives have emerged, namely sclerotherapy with safer and more effective sclerosing agents, raising the need of reassessing the comparison of different office-based procedures. There are also patients with special conditions such as pregnancy, bleeding disorders, immunosuppression, inflammatory bowel disease, and liver cirrhosis, which require a targeted and specific approach [1]. Regarding these groups there is still little information on the efficacy and safety of these approaches.
In this systematic review and meta-analysis, our aim was to compare the efficacy and safety of sclerotherapy and RBL, as these are the two most performed procedures in daily practice.
Methods
Search and Selection
This systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [5]. We included fully published randomized controlled trials and prospective cohort studies including patients with HD submitted to the non-surgical treatments sclerotherapy and RBL, and evaluating efficacy and safety outcomes (detailed below).
Study search was performed through scanning of three electronic databases: MEDLINE through PubMed, ISI Web of Knowledge, and Scopus Preview, from inception to March, 2021.
The following search query was used for PubMed: ((hemorrhoid) OR (haemorrhoid) OR (”hemorrhoidal disease”) OR (”haemorrhoidal disease”)) AND ((band ligation) OR (ligation) OR ”rubber band))) AND (”sclerotherapy” OR polidocanol). The search terms for other databases were adapted from this query. Additional studies were identified by checking the list of references of all included studies and also review articles on this topic.
After removal of duplicates, two authors (P. Salgueiro and M.I. Ramos) independently screened all titles and abstracts for relevance. The full text of relevant studies was then evaluated by the same researchers to apply the inclusion/exclusion criteria described below. Disagreements among the two authors were solved by intervention of a third investigator (D. Libânio).
This systematic review was registered at International Prospective Register of Systematic Reviews (PROSPERO) with the identifier CRD42021275047.
Inclusion/Exclusion Criteria and Outcomes
We included studies enrolling patients with symptomatic HD of any grade undergoing RBL or sclerotherapy, without distinction for age or gender. Only studies comparing RBL and sclerotherapy were included. All types of sclerosant (ethanolamine + almond oil, polidocanol, dextrose, etc.) and also endoscopic or anoscopic techniques were considered. Regarding RBL, neither the type of instrument used for the application nor the number of rubber bands applied were exclusion criteria.
For being included in this systematic review, the studies had to report at least one of the following outcomes: for efficacy, we considered overall control of symptoms, hemorrhoidal prolapse reduction (according to Goligher score), bleeding control, pain relief, patients’ satisfaction, and disease recurrence; regarding safety, complications related to the office-based procedures, such as pain and bleeding, were assessed. Length of follow-up was not an exclusion criteria.
Pain assessments differ between studies with the use of different scores (VAS-scale, Numeric Pain Rating Scale, and Wong Baker scale); therefore, for the analysis, pain as a HD symptom, was categorized into a dichotomous output: present or absent. We excluded from the meta-analysis studies that only reported the average pain, based on the chosen score.
As reports of patients’ satisfaction also differ between studies, this outcome was categorized into a dichotomous output: cured/improved (symptom free or mild residual symptoms but not requiring further treatment) or unchanged/worse (no symptom improvement and/or requiring further treatment).
Quality Assessment
Quality evaluation of included studies was performed through consensus by M.I. Ramos and D. Libânio using the Cochrane risk of bias tool for randomized studies and Newcastle-Ottawa Scale (NOS) for prospective cohort studies. Cochrane risk of bias tool is based on 7 domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Studies were classified into high risk or low risk of bias. Trials with low risk of bias were considered as high-quality trials. NOS is based on a “star system,” in which a study is judged on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of outcome of interest for cohort studies. The total maximum score is 9 and a study with a score from 7 to 9 has high quality.
Data Extraction and Analysis
P. Salgueiro and M.I. Ramos extracted data from all the included studies, which were analyzed for the above methodological quality and for details regarding participants, interventions, and outcomes. Data entry was performed by M.I. Ramos and checked by P. Salgueiro and D. Libânio. Relative risks (RR) along with 95% confidence intervals (CI) were calculated for the dichotomous outcomes. Pooled RRs were then calculated using Review Manager (RevMan Version 5.4.1). Heterogeneity was evaluated with the Cochran’s Q test and I 2. Significant heterogeneity was defined as I 2 > 50% or Cochran’s Q test p < 0.05. Subgroup analysis was planned according to inclusion of different grades of hemorrhoidal disease (grade II only vs. grade I-III), according to the sclerosant used and study design and quality. In case of significant heterogeneity, sensitivity (leave-one-out meta-analysis) was performed to explore the reasons for heterogeneity.
Results
Description of Studies
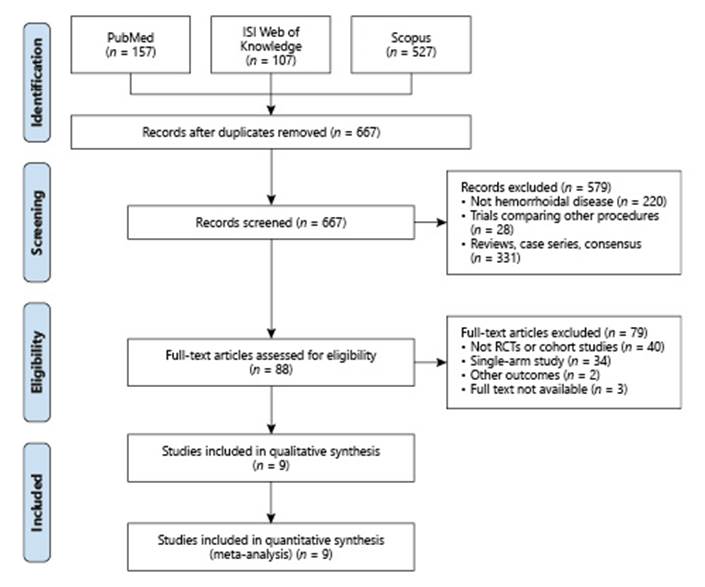
A total of 791 records were identified in PubMed (n = 157), ISI Web of Knowledge (n = 107), and Scopus (n = 527). After exclusion of duplicates, 667 were screened for relevance and 88 were assessed for full-text eligibility. Nine studies comparing RBL with sclerotherapy met the inclusion criteria (Fig. 1).
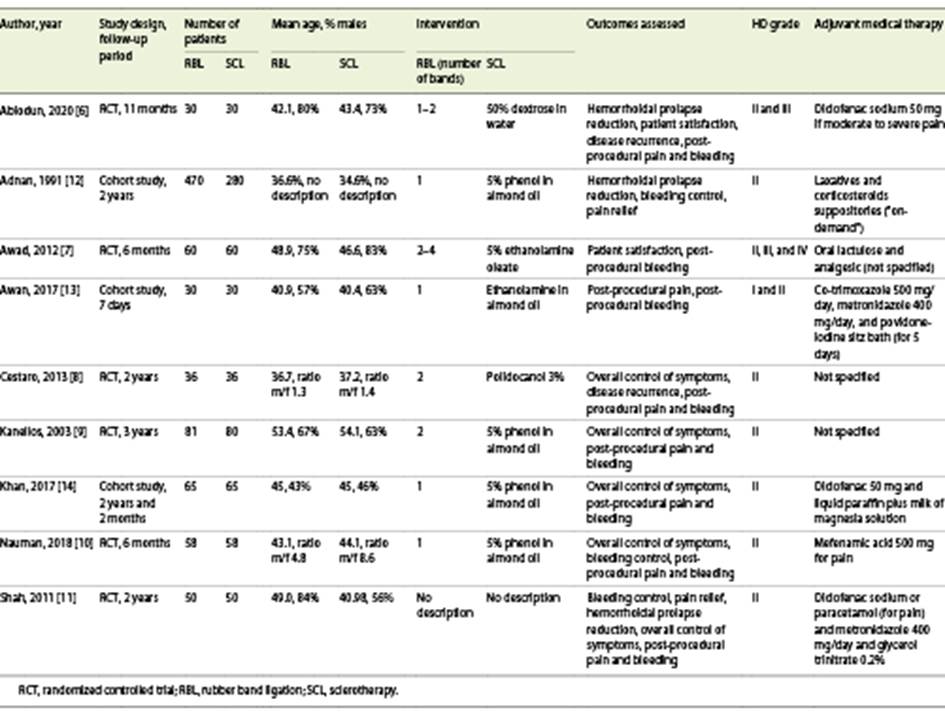
Of these, six [6-11] were RCTs and three [12-14] were prospective cohort studies. Baseline characteristics of the included studies are displayed in Table 1.
All the studies compared patients with grade II hemorrhoids, but some of them also included grades I and/or III and/or IV [6, 7, 13] according to Goligher Prolapse Score. Six studies [8-12, 14] only included patients with grade II hemorrhoids.
In the sclerotherapy group, different sclerosants were used: dextrose in water (1 study [6]), phenol in almond oil (4 studies [9, 10, 12, 14]), ethanolamine in almond oil (2 studies [8, 9]), and polidocanol (1 study [8]). In the RBL group, one or more bands were used: one band (4 studies [10, 12-14]), two bands (2 studies [8, 9]), one or two (1 study [6]), two to four (1 study [7]).
Regarding risk of bias, 2 [12, 14] of the 3 cohort studies were classified as high-quality articles according to the Newcastle-Ottawa Quality Assessment Scale (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000522171). The results of the RCTs [6-11] are favorable, with a low risk of bias (online suppl. Fig. 1). However, there is a high risk of performance bias since the blinding of participants and medical staff was not possible in these studies (online suppl. Fig. 2).
Outcomes
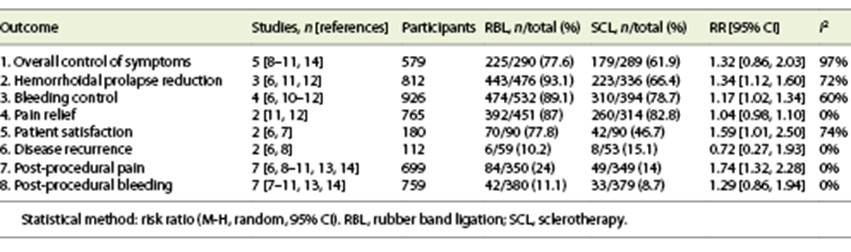
Table 2 includes all the outcomes. Online supplementary Table 2 includes analysis by subgroups according to the HD grade (grade II and other grades versus RBL), in the outcomes in which this sub-analysis was possible to perform (post-procedural pain and bleeding).
Online supplementary Table 3 includes a subanalysis according to the type of injected sclerosant (phenol in almond oil and other sclerosants versus RBL). It was possible to compare the efficacy outcomes “overall control of symptoms” and “bleeding control” and the safety outcomes “post-procedural pain” and “post-procedural bleeding.”
Efficacy Outcomes
Both RBL and sclerotherapy had similar efficacy in overall control of HD symptoms (RBL 77.6% vs. sclerotherapy 61.9%, RR 1.32, 95% CI 0.86-2.03) (Fig. 2a). However, if Kanellos, 2002 [9] and Khan, 2017 [14] (cohort study and RCT with high risk of bias, respectively) are excluded, heterogeneity reduces from 97% to 0% and RBL is associated with a significantly higher control of HD symptoms (RBL 83.3% vs. sclerotherapy 68.8%, RR 1.17, 95% CI 1.05-1.31).
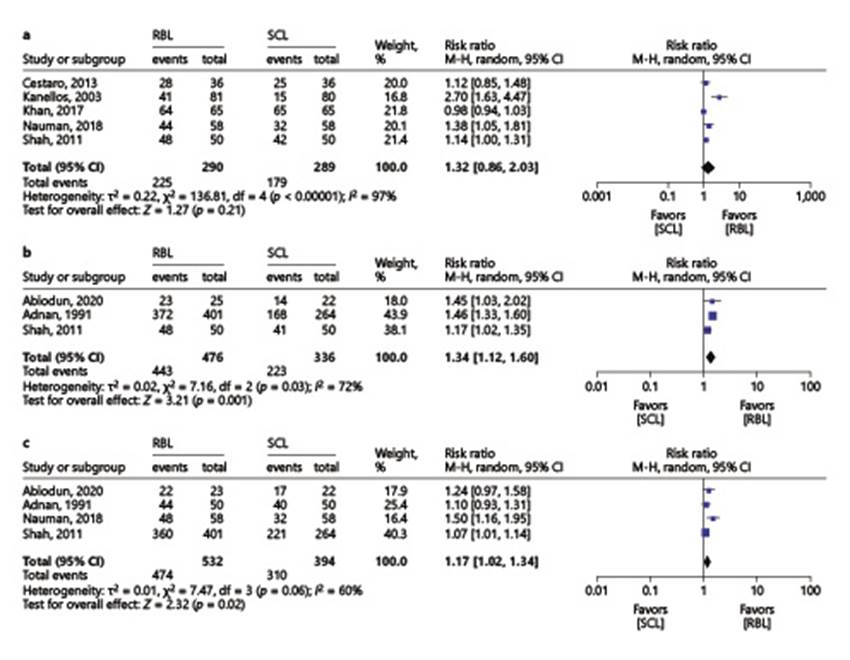
Fig. 2 Comparison 1: rubber band ligation versus sclerotherapy for hemorrhoidal disease; outcome 1: overall control of symptoms (a); outcome 2: prolapse reduction (b); outcome 3: bleeding control (c).
Prolapse reduction was significantly better with RBL vs. sclerotherapy (93.1% vs. 66.4%, RR 1.34, 95% CI 1.12-1.60) (Fig. 2b). Excluding Shah, 2011 [11], an RCT that did not report the number of bands nor the type of sclerosant used, heterogeneity decreases from I 2 of 72% to 0%, without significant alteration in the pooled estimate (RBL 92.7% vs. sclerotherapy 63.6%, RR 1.46, 95% CI 1.33-1.60). Bleeding control was also significantly higher with RBL (89.1% vs. 78.7%, RR 1.17, 95% CI 1.02-1.34) (Fig. 2c). Regarding pain relief there was no significant difference between the two interventions (RBL 87% vs. sclerotherapy 82.8%, RR 1.04, 95% CI 0.98-1.10) (online suppl. Fig. 3).
Patient satisfaction was significantly higher with RBL (77.8% vs. 46.7%, RR 1.59, 95% CI 1.01-2.50, I 2 = 74%) (Fig. 3).
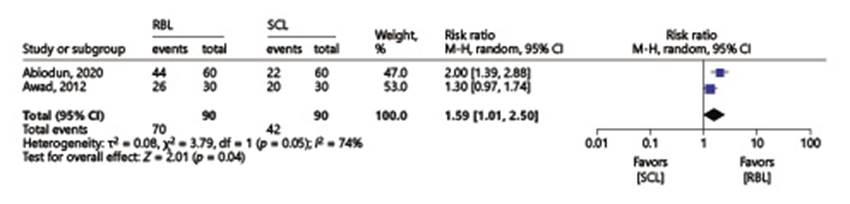
Fig. 3 Comparison 1: rubber band ligation versus sclerotherapy for hemorrhoidal disease; outcome 5: patient satisfaction.
The risk of disease recurrence at 3 months was similar between the two groups (RBL 10.2% vs. sclerotherapy 15.5%, RR 0.72, 95% CI 0.27-1.93) (online suppl. Fig. 4).
Concerning the efficacy outcomes, it was not possible to make subgroup analysis by HD grade. Yet, it is important to mention that the studies included in the “overall control of symptoms” and “pain relief” analysis enrolled only patients with grade II HD.
In the other subgroup analysis, according to the type of sclerosant, it was possible to compare the efficacy outcomes “overall control of symptoms” and “bleeding control.” Regarding overall control of symptoms, there was no significant difference between the phenol in almond oil subgroup and RBL (RBL 73% vs. phenol sclerotherapy 55.2%, RR 1.53, 95% CI 0.38-6.20). However, RBL was significantly better than the “other sclerosants” subgroup (88.4% vs. 77.9%, RR 1.14, 95% CI 1.01-1.28) (online suppl. Fig. 5). It is important to highlight that the results were quite heterogeneous in the phenol in almond oil group (I 2 = 99%). There was no difference between RBL and each subgroup concerning bleeding control (online suppl. Fig. 6).
In the remaining outcomes, subgroup analysis according to study design/study quality was not possible.
Safety Outcomes
The risk of post-procedural pain was significantly higher with RBL (24% vs. 14%, RR 1.74, 95% CI 1.32-2.28) (Fig. 4a). However, there was no significant difference between the two interventions regarding post-procedural bleeding (RBL 11.1% vs. sclerotherapy 8.7%, RR 1.29, 95% CI 0.86-1.94) (Fig. 4b).
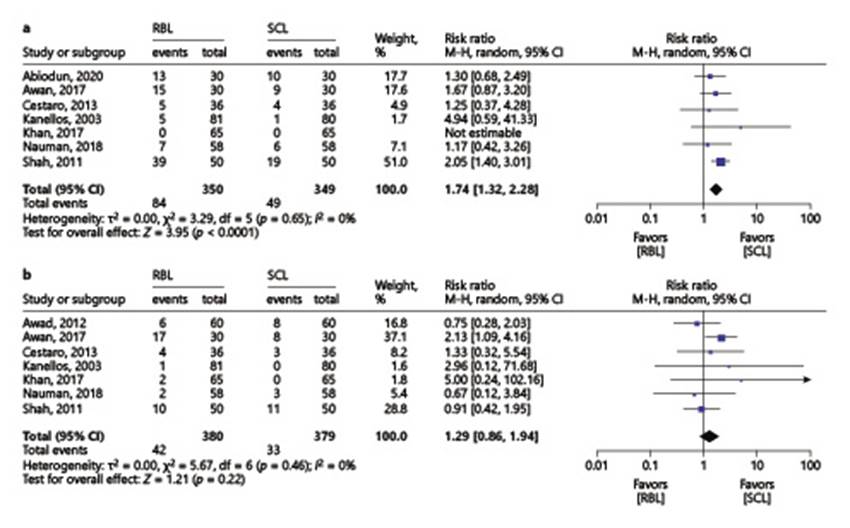
Fig. 4 Comparison 1: rubber band ligation versus sclerotherapy for hemorrhoidal disease; outcome 7: post-procedural pain (a); outcome 8: post-procedural bleeding (b).
In the subgroup analysis according to HD grades, post-procedural pain was higher with RBL (19.3 % vs. 10.4%, RR 1.90, 95% CI 1.35-2.67) in “grade II HD” subgroup and there was no significant difference between the two interventions in the “other grades” subgroup (RBL 46.7% vs. sclerotherapy 31.7%, RR 1.47, 95% CI 0.93-2.33) (online suppl. Fig. 7). With regard to post-procedural bleeding, there were no significant differences in the two subgroups (online suppl. Fig. 8).
At last, in the type of sclerosant subgroup analysis, we observed that, regarding post-procedural pain, RBL was similar to phenol in almond oil (RBL 5.9% vs. phenol sclerotherapy 3.4%, RR 1.78, 95% CI 0.48-6.57). Nevertheless, when comparing “other sclerosants” subgroup with RBL, post-procedural pain was more common in the RBL group (49.3% vs. 28.8%, RR 1.76, 95% CI 1.32-2.34) (online suppl. Fig. 9). Concerning post-procedural bleeding, there were no significant differences between RBL with either phenol in almond oil or other sclerosants (online suppl. Fig. 10).
Discussion
This systematic review and meta-analysis compared the efficacy and safety of the most commonly performed office-based procedures in the treatment of HD, RBL and sclerotherapy. We found that RBL is associated with a better overall control of symptoms, namely hemorrhoidal prolapse and bleeding, but at the expense of higher post-procedural pain. Despite this higher incidence of pain after the procedure, patients undergoing RBL are more satisfied with this treatment than those treated with sclerotherapy. These findings suggest that RBL should be the first-line office-based procedure for patients with HD.
The range of treatment options for hemorrhoidal disease can vary and they are divided into conservative measures, office-based procedures, and surgical treatments.
Lifestyle changes, dietary changes, laxatives and phlebotonic medications and topical anti-inflammatory drugs are effective in controlling HD symptoms in the short term. Since these measures produce beneficial effects, they should be implemented in all patients with HD [15].
The minimally invasive office-based procedures are alternatives to the traditional hemorrhoidectomy and hemorrhoidopexy for symptomatic patients with low-grade HD, especially because of higher rate of surgical complications. In this way, surgical treatment should be reserved for refractory cases, grade IV, or mixed HD [16].
The instrumental office-based treatment is usually indicated for hemorrhoidal disease grade I and II [17], though it can also be used in grade III hemorrhoidal disease [18].
RBL is usually the preferred office-based treatment for grades I to III hemorrhoids because of its effectiveness when compared with other office-based procedures [16, 19]. This technique is performed using an anoscope or an endoscope in retroversion in the patient’s rectum and consists in positioning elastic bands above the dentate line to strangulate the hemorrhoidal piles, resulting in ischemia and subsequent necrosis of the prolapsed mucosa. It is a fast, easy-to-learn, and well-tolerated procedure [20].
Bleeding and pain are among the most frequent complications of RBL [21, 22]. Post-ligation bleeding typically occurs 10 to 14 days after treatment, but it may occur immediately after the procedure [15]. The risk of bleeding is more significant in patients under antiplatelet or anticoagulation medications, so it is not indicated in this subgroup of patients [23]. Although RBL may be more painful historically, the differences to other office-based treatments are smaller in more recent studies [24].
Hemorrhoidal sclerotherapy is a procedure commonly used to treat grade I and II hemorrhoidal disease [25, 26]. It has also been used in internal grade III hemorrhoids [20] although, in these cases, there is little scientific evidence supporting its efficacy [22]. The hemorrhoidal injection with sclerosant agents interrupts the vascular blood supply and leads to scarring, which prevents further bleeding and prolapse of the hemorrhoidal tissue [27, 28].
Many sclerosant agents have been used over time. Sclerotherapy with older sclerosing agents seems to be less effective than RBL, which is why some authors recommend that this technique, at least with those sclerosing agents, should only be used in grade I HD [19]. More recently, the sclerosing substance polidocanol started to be employed in the form of foam [29]. The foam formulation allows for greater efficacy and use of lower doses of sclerosing agent [30]. Although it is a sclerosing substance with very promising results, data comparing polidocanol foam with other hemorrhoidal disease ablative techniques is lacking. Since there are no comparative studies between RBL and polidocanol foam sclerotherapy, none of the studies included in this meta-analysis used polidocanol foam as a sclerosing agent.
The most common complications of sclerotherapy include mild anal discomfort and bleeding. However, the bleeding risk is often described as being inferior to that observed with RBL [31].
A previous meta-analysis compared various HD treatment modalities [31]. The outcomes evaluated included response to therapy, need for further therapy, and complications. In that review, patients treated with RBL were less likely to require further therapy than those treated with sclerotherapy, although pain was significantly more likely to occur following RBL. Therefore, it was concluded that RBL was better than sclerotherapy in response to treatment for all hemorrhoids, so RBL was recommended as the initial treatment for grades I to III HD [4].
Twenty-six years later, in this systematic review, we have expanded the outcomes by including patient satisfaction and discriminating the effect of the office-based procedures in the most frequent HD symptoms (prolapse, bleeding, and pain). It is also important to mention that the studies included in our meta-analysis are different from those included in the previous one.
The control of the hemorrhoidal symptoms is the most obvious measure of success for any procedure, so it is a very important and relevant aspect when choosing the primary treatment. In our review, both RBL and sclerotherapy were effective in controlling overall HD symptoms; however, control of prolapse and bleeding was significantly better with RBL. Pain relief was equally effective with both techniques.
The safety of the interventions is also an extremely important aspect, particularly when dealing with a benign disease such as HD. In this situation, the occurrence of serious complications is especially unwanted and unacceptable. Post-procedural complications, such as pain and bleeding, are crucial factors that can influence a patient’s decision to accept or not a specific type of treatment. In the present study, the risk of post-procedural pain was greater with RBL; however, in the group of grade II HD, the results were similar.
Recurrence was similar with both procedures and was less than 20%.
Finally, patient satisfaction is determined by the efficacy and safety of each procedure and, in our study, was higher with RBL.
This systematic review and meta-analysis has some limitations. First, the lack of standardization of therapies concerning the number of bands used in each session, the type and volume of the injected sclerosant, the number of hemorrhoidal cushions treated in each session, as well as the adjuvant medical therapy (not always specified in all studies included, see Table 1) could contribute to the heterogeneity of the results. Second, significant heterogeneity was found in some outcomes, which can be explained by the type of sclerosant used: phenol in almond oil subgroup was associated with 99% heterogeneity in the outcome “overall control of symptoms” and with 77% heterogeneity in “control of bleeding.” Also, when we performed subgroup analyses, for some of the outcomes, it was possible to include only a small number of studies. Third, even though we are aware of the importance of including studies published only as conference abstract, we decided not to do so since, in most abstracts, perhaps due to restrictions on the number of words allowed, extractable data (inclusion/exclusion criteria, technique used, type of sclerosant, etc.) are rarely reported for carrying out meta-analysis. Lastly, in most centers, as in ours, it is usual to refer grade IV HD patients directly to surgical treatment. In our meta-analysis, since the grade of hemorrhoidal disease was not considered an exclusion criterion, among the included studies, one included patients with grade IV HD [18]. It refers to a randomized trial including patients with liver cirrhosis and hemorrhoidal disease grades II to IV that compared RBL and sclerotherapy (60 patients included in each therapeutic arm). Patients with grade IV HD included in that study represent only 2.5% of the sample (2 patients in the ligation group and 1 patient in the sclerotherapy group); therefore, we do not believe that the inclusion of participants with such an advanced HD had a significant influence on the results obtained.
Additionally, it is important to mention that in our research we did not find comparative studies between RBL and the most recent and promising sclerosing agents polidocanol foam and aluminum potassium sulfate and tannic acid. If, at the time of our research, comparative studies with these sclerosing agents were published, their inclusion in the meta-analysis could possibly influence the results in terms of benefiting sclerotherapy. At this time, it is not possible to draw conclusions about comparing RBL with these new sclerosing agents.
Conclusion
RBL is currently the best office-based treatment for HD grades I to III since it is more effective than sclerotherapy with regard to overall control of HD symptoms, specifically prolapse and bleeding. Despite the higher incidence of pain after performing RBL, patients undergoing this technique have higher rates of satisfaction than those treated with sclerotherapy. Recurrence is similar for both procedures.
While waiting for the publication of comparative trials with new sclerosants, RBL remains the office-based treatment of choice.