Introduction
Varices are abnormally large portosystemic venous collaterals most commonly recognized near the gastroesophageal junction. Varices that appear in other gastrointestinal locations are called ectopic. Although unusual, they can account for up to 5% of all variceal hemorrhages [1].
Parastomal varices (PV) usually occur in ostomized patients with chronic liver disease (CLD) and emerge at the mucocutaneous junction of the stoma. PV develop due to a portosystemic shunt between the portal circulation of the bowel and systemic circulation of the abdominal wall. There are no pathognomonic physical symptoms or signs of PV. A raspberry appearance of the stoma with visibly dilated submucosal veins and bluish discoloration and hyperkeratosis of the surrounding skin have been used to describe PV [2].
Doppler ultrasound, computed tomography (CT) and magnetic resonance angiography may identify varices in the region of the stoma and facilitate the diagnosis of CLD, portal hypertension and the assessment of portal patency [3].
Parastomal variceal bleeding (PVB) tends to present as chronic and recurrent rather than massive bleeding, although the need for a blood transfusion is expected in 42.9% [4]. The mortality rate of PVB is estimated at around 3-4% [2, 3]. Despite the low mortality rate, given its insidious but recurring nature, greater awareness and an established therapeutic strategy will certainly be useful.
Case Presentation
A 63-year-old man presented to the emergency department several times throughout 1 year with self-limited bright red blood in his colostomy bag. The patient had undergone abdominoperineal resection (with permanent colostomy) due to rectal carcinoma 4 years earlier. Initially, stoma bleeding due to local trauma was presumed. In the majority of those episodes, no bleeding source was identified and the patient was discharged with the indication to perform a colonoscopy in an outpatient setting. On 2 occasions, a stoma bleeding site was identified and local approaches including direct compression, silver nitrate application and suture ligation were applied with transient success. However, recurrence of hemorrhage ensued, requiring inpatient admission for transfusion and additional evaluation. Physical examination evidenced stigmata of CLD and alcoholism, pallor and a raspberry appearance of the stoma with dilated submucosal veins (Fig. 1). Initial laboratory analysis revealed hemoglobin of 6.8 g/dL, platelets 53,000/mm3 and liver function tests compatible with CLD (Child-Pugh B, MELD-Na 16). Upper endoscopy and colonoscopy were normal.
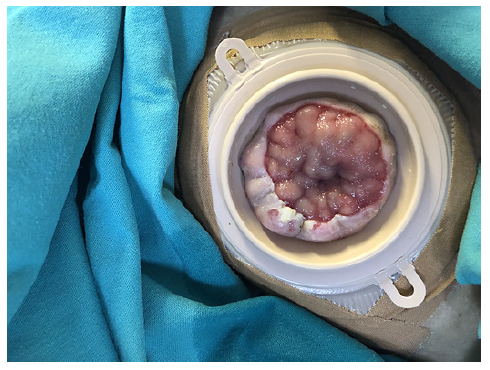
Fig. 1 Colostomy stoma, with visibly dilated submucosal veins and keratosis of the colon mucosa around it.
An abdominal CT showed features suggestive of cirrhosis and collateral venous circulation originating from the inferior mesenteric vein, insinuating itself in the neck of the stoma. A peristomal varicose conglomerate was observed in the thickness of the abdominal wall, giving rise to multiple varicose veins. Many of the varices were converging in the stoma, and others were running through the thickness of the lower abdominal wall, draining distally into the left common femoral vein (LCFV) (Fig. 2). The portal vein was patent.
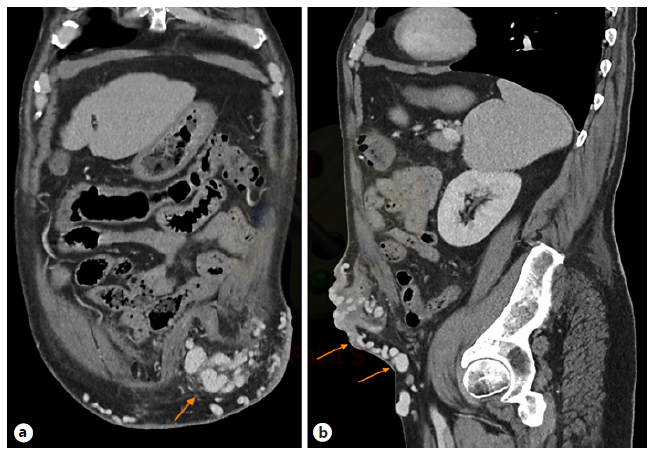
Fig. 2 Intravenous contrast-enhanced CT coronal (a) and sagittal (b) images of the abdomen with multiple varices (arrows) within the left lower quadrant stoma.
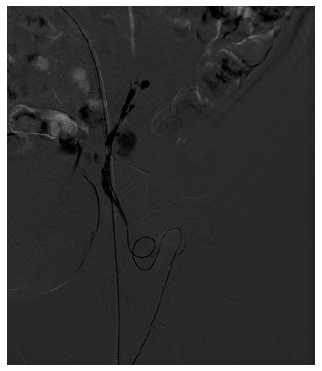
Due to an episode of acute spurting bleeding with hypovolemic shock during hospital admission, the patient was initiated on terlipressin and agreed to be submitted to a balloon-occluded retro-grade transvenous obliteration (BRTO) procedure. Through the right femoral approach, a balloon catheter was placed at the portosystemic shunt drainage site in the LCFV. A 6-F guiding sheath at the left external iliac vein was used for extra catheter support. After balloon inflation, a retrograde transvenous venogram from the systemic venous side of the system was performed, demonstrating multiple parastomal systemic draining veins (Fig. 3). A 2.7 F × 130 cm Progreat microcatheter (Terumo, Tokyo, Japan) was used for distal (rather proximal from a blood flow standpoint) selective catheterization of these efferent systemic draining veins (Fig. 4). N-butyl-2-cyanoacrylate embolization of PV was performed (Fig. 5). Postembolization control evaluation revealed exclusion of varicose drainage in the LCFV. The procedure proceeded uneventfully, with bleeding resolution.
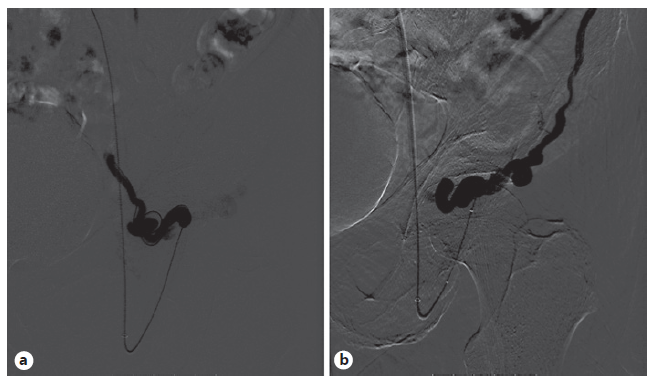
Fig. 3 Retrograde transvenous venogram from the systemic venous side of the system showing multiple parastomal systemic draining veins coursing along the medial (a) and lateral (b) aspect of the stoma.
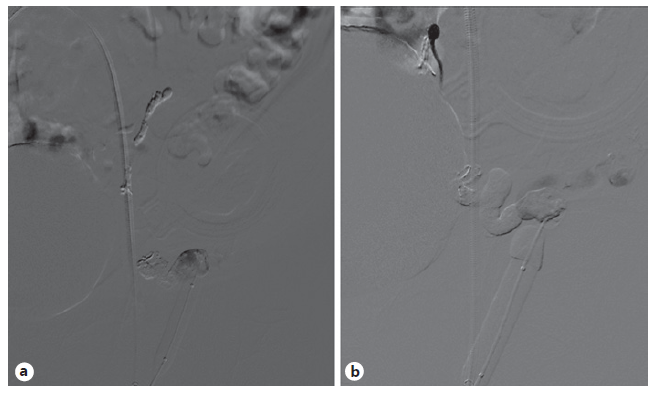
Fig. 5 a Venogram taken near the end of the “BRTO approach”, after glue embolization. b Final venogram showing exclusion of varicose drainage into the left femoral vein.
The case was subsequently discussed in a multidisciplinary team meeting. Considering the preeminence of PV, with multiple portosystemic shunts, it was concluded that local obliteration of the remaining collaterals assisted by endoscopic ultrasound alone was not feasible. The patient was then proposed for a transjugular intrahepatic portosystemic shunt (TIPS) conjugated with percuta-neous transhepatic obliteration (PTO).
Fearing possible complications, such as the development of hepatic encephalopathy (HE), the patient refused the procedure at first. Nevertheless, 7 days after BRTO, following terlipressin suspension and initiation of propranolol, the patient experienced a new episode of self-limited PVB and agreed to undergo the proposed treatment. Through the right internal jugular vein access, the right suprahepatic vein was selected and shunted with the right branch of the portal vein. Upon tract balloon dilation, an 8-10 mm × 5 cm Viatorr TIPS prosthesis (W.L. Gore, Flagstaff, AZ, USA) was placed, resulting in a hepatic-venous pressure gradient (HVPG) reduction from 20 to 14 mm Hg. After selective catheterization of the inferior mesenteric vein, an anterograde transvenous venogram was performed, demonstrating multiple pericolostomy varicose veins. In phlebographic control, the pericolostomy varicose plexus was selected, with embolization of the main varix with coils (Fig. 6). The procedure proceeded uneventfully.
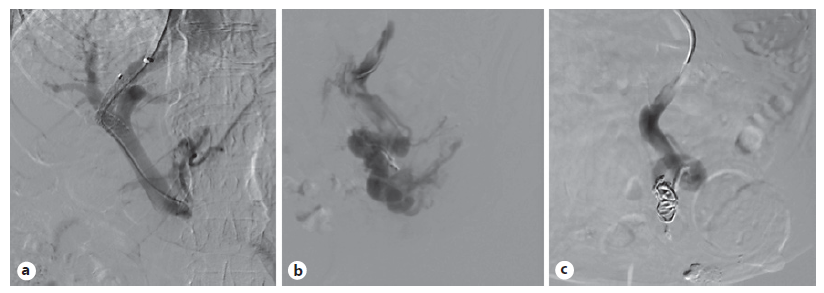
Fig. 6 a Transhepatic approach after TIPS prosthesis has been placed. b Stoma varicose plexus being selected. c Embolization of the main varix with coils.
Four months after the procedure, the patient presented with grade II HE in a routine hepatology consultation. Nevertheless, it was successfully managed with medical therapy (lactulose and rifaximin).
After a 9-month follow-up, the patient has remained well with-out further episodes of PVB or other adverse effects.
Discussion
Significant parastomal bleeding must raise suspicion of portal hypertension and underlying CLD, which have to be actively excluded. The management of PVB should involve a multidisciplinary approach (with hepatologists, interventional radiologists and surgeons), with a progressive escalade in more invasive methods when local procedures are not effective. Concomitant treatment of CLD should be carried out, particularly removing the etiological factor(s) causing liver injury whenever possible [3, 4].
Simple local procedures, such as pressure dressings, epinephrine-soaked gauze, gel foam, and suture ligation have been used with success on the initial bleeding episode. However, bleeding recurrence is the rule [2-4].
Some treatments have shown considerable morbidity and/or recurrence, not being valuable options in the management of this pathology. These include sclerotherapy, which resulted in stomal damage and/or recurrent bleeding in nearly all patients [5]. Mucocutaneous disconnection and surgical relocation of the stoma were also associated with recurrent bleeding and significant peri-operative surgical risk [6].
From a pathophysiological point of view, pharmaco-therapy used in gastroesophageal varices management may be applied in patients with PVB by reducing HVPG [7]. However, data on the role of medical therapy in PVB is scarce. β-Blockers have presented conflicting results: older studies showed that they are not effective in PVB [4], while a few recent clinical reports showed they may delay their recurrence [8]. In 2 patients with contraindications for intravascular procedures, octreotide showed to be effective as a palliative care option, without significant side effects, suggesting it can be considered for patients for whom noninterventional care is indicated [9].
Minimally invasive endovascular techniques guided by ultrasound or CT have been safely used in the management of PVB. The simplest and least invasive procedure is direct ultrasound-guided percutaneous embolization with cyanoacrylate or coils. Nevertheless, this technique showed better results when a single dominant varix is identified and has an increased risk of embolization glue migration and mucosal damage at the stomal site. A proposed way to minimize glue migration is to combine this modality with ultrasound-guided systemic venous compression [10, 11].
The BRTO approach from the systemic venous side and PTO approach from the portal venous side are other endovascular techniques angiography-guided, which have been proving to be effective and safe. The main limitation to these procedures is long-term recurrence due to failure to embolize all feeding vessels, or due to the rapid development of new vessels [11-14].
Although embolization guided by endoscopic ultra-sound has been tested successfully in PVB [15], minimal length necessity of intubation (which would make maintaining the endoscope position difficult) and the easier percutaneous approach make this modality unattractive [16].
Hypothetically, surgical portosystemic shunt can be considered as a decompressive measure [4]. However, given the increased morbidity, lower efficacy and inadequacy in transplant candidates, TIPS is clearly a preferred option. TIPS is by far the best-studied modality for managing PVB, as it ultimately reduces HVPG. Some limitations of TIPS are its contraindication in liver neoplasia and the risk of developing HE in advanced CLD (although it can usually be managed with medical treatment, as occurred in our patient). Moreover, even though it appears to be the most effective treatment modality, up to 25% of patients develop rebleeding. Although the common understanding has been that varices rarely bleed at HVPG less than 12 mm Hg, there were several cases of rebleeding by PV after TIPS despite lower HVPG [17, 18]. This statement highlights the importance of other coadjuvant treatments such as BRTO or PTO, particularly in cases with higher HVPG, as was the case in our patient. These two modalities can be performed following bleeding recurrence after TIPS, when TIPS is contraindicated or, as in this case, to complement TIPS before and during this procedure.