Introduction
Endoscopic submucosal dissection (ESD) is a well-established treatment for colorectal lesions, enabling en bloc resection regardless of a lesion’s size and morphology and allowing precise assessment of histological curability [1, 2]. However, its implementation in Western countries has been slow and challenging, mainly due to the long learning curve required, higher risk of associated adverse events, lack of structured training programs, few suitable starting cases in the stomach, and lack of experts [3, 4]. As a result, only a handful of centers in Europe have established comprehensive and proficient ESD programs.
When compared to the stomach, colorectal ESD remains technically challenging due to anatomical features of the colon, including thin walls, narrow lumen, and the presence of peristalsis, which are associated with difficulties on the endoscopic maneuverability.
Besides these anatomical features, several studies have shown that submucosal fibrosis, tumor size and location, and paradoxical movements of the endoscope were also related to procedure difficulty during colorectal ESD [5-7]. Severe submucosal fibrosis can complicate the identification of the appropriate submucosal layer and its separation from the muscular layer and has been linked to incomplete resection, lengthy procedure time, and higher rate of perforation [7-12]. It has been related to prior biopsy or tattooing, residual or recurrent lesions, chronic inflammation (such as ulcerative colitis), and tumor invasion of the submucosal layer [12-19]. Simultaneously, large tumor size, lesions across the fold, protruding morphology, nodular-mixed granular lateral spreading tumors (LST G-M), and non-granular pseudo-depressed type LST (LST NG-PD) have inconsistently been identified as preoperative predictors of severe fibrosis [8, 14, 19, 20].
These observations have been obtained from expert centers in Asia, and data on the effect of severe fibrosis on the results of rectal ESD performed in the West, where endoscopic mucosal resection (EMR) is widely performed (predictably resulting in lesions with profound submucosa fibrosis), are lacking.
Therefore, we conducted a retrospective study to compare outcomes of rectal ESD between lesions with severe fibrosis and lesions without severe fibrosis in a European center and examined the learning curve in lesions with severe fibrosis.
Patients and Methods
Patients and Lesions
We reviewed the records of 195 lesions in 192 consecutive patients with rectal neoplasms referred to ESD at our tertiary center at Centro Hospitalar de Lisboa Ocidental between January 2013 and January 2021. All patients had been informed about the risks and benefits of ESD and provided written informed consent.
All ESDs were performed by the same expert endoscopist (P.B.), and lesions were investigated by white light and narrow-band imaging to detect signs of invasive cancer and to assess the most optimal endoscopic resection technique. The endoscopic appearance of the lesions was classified according to the Paris endoscopic classification, and tumors were macroscopically classified as protruding tumors (0-Is) or 1 of the 4 sub-types of LST: LST granular homogeneous (LST G-H), LST G-M, LST non-granular flat-elevated (LST NG-F), and LST NG-PD [21, 22].
Indications for rectal ESD were described in a previous study of our center and were adapted from those proposed by the Colorectal ESD Standardization Implementation Working Group and in accordance with the European Society of Gastrointestinal Endoscopy (ESGE) [23-25]. Those included LST NG ≥20 mm, LST G (mixed type) ≥40 mm, depressed tumors ≥20 mm, lesions with type 2B classification of the Japan Narrow Band Imaging Expert Team (JNET) Classification, and lesions that otherwise cannot be optimally and radically removed by snare-based techniques (i.e., lesions located near or at the dentate line, those with non-lifting sign, prior failed EMR, sporadic localized tumors in chronic inflammation) [26]. Neuroendocrine tumors and subepithelial lesions were excluded.
ESD Procedure
According to the expected procedure time and difficulty, procedures were performed by using conscious sedation (with midazolam and fentanyl), deep sedation, or general anesthesia with endotracheal intubation, at the discretion of the endoscopist and anesthesiologist (if involved). Patients were monitored with continuous electrocardiographic registration, pulse oximetry, and noninvasive blood pressure measurement.
With slight variations, rectal ESD was performed in accordance with our previous report [23]. A single-channel gastroscope (GIF-HQ190; Olympus America, Inc., Center Valley, PA, USA) with a disposable distal attachment (D-201; Olympus, Tokyo, Japan) on the tip and carbon dioxide insufflation were used. DualKnife (KD-650L; Olympus) was routinely used in combination with ITknife nano (KD-612L; Olympus) until the end of June 2017, while from July 2017, the endoscopist mostly used a FlushKnife BT (1.5 mm) (Fujinon-Toshiba ES System Co., Omiya, Japan). The electrosurgical ERBE ICC 200 generator unit (ERBE Elektromedizin, Tubingen, Germany) was set at “Endocut” (effect 3, 60 W) for mucosal incision, “Forced Coag” (45-55 W) for submucosal dissection, and “Soft Coagulation” for hemostasis (45-55 W). During submucosal dissection of areas with severe fibrosis, “Endocut” was used at the discretion of the endoscopist. Submucosal injection was performed using a mixture of 4% gelatin solution (Gelofundin 4%, B. Braun Melsungen AG, Germany), indigo carmine nad adrenaline (1:250,000). Hemostatic forceps (Coagrasper, FD-411UR, Olympus) were used if hemostasis by the knife in use was ineffective.
When the presence of severe submucosal fibrosis was predicted, the tunneling technique was performed ad initium. Whenever submucosal fibrosis was not predicted and was encountered during the procedure, different strategies were used, including tunneling technique, pocket-creation method, or rarely clip with line to assist traction, which have been described elsewhere [27-29]. In cases where ESD had to be abandoned, a conversion to EMR (Hybrid ESD) was considered.
Classification of Fibrosis
Submucosal fibrosis was evaluated based on findings obtained at the time of submucosal injection and dissection. The degree of submucosal fibrosis was classified into 3 types (F0-2): F0 - no fibrosis, which is demonstrated as a blue transparent layer; F1 - mild fibrosis, which appeared as a white web-like structure in the blue submucosal layer; and F2 - severe fibrosis, which appeared as a white muscular-like structure without a blue transparent layer in the submucosal layer (Fig. 1) [8].
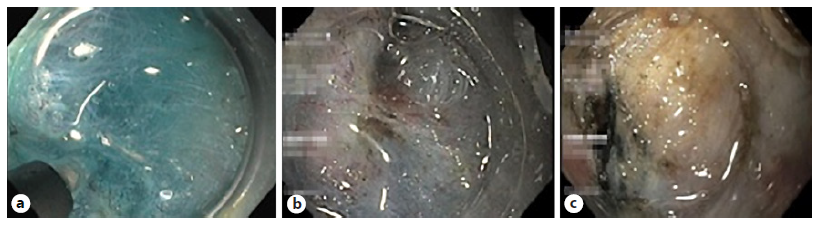
Fig. 1 Degree of fibrosis of the submucosal layers according to appearance of the layers during submucosa injec-tion. a F0. b F1. c F2.
Patients were divided into F0/F1 (nonsevere fibrosis) and F2 (severe fibrosis) groups.
Histopathology Assessment
The resected tissue specimens were pinned on cork after removal and fixed using 4% neutral buffered formalin, before being sent to the pathology department. In cases of piecemeal resections, if possible, the specimen was reconstructed by appropriate fixation onto cork. Histological evaluation was performed in accordance with ESGE guidelines [25, 30].
Outcome Measures and Definitions
Procedure time was defined as the time from mucosal incision to complete removal of the lesion. Resection speed was defined as square millimeter resected per minute (mm2/min), calculated from the surface area (specimen diameter in long axis × specimen diameter in short axis × π × 0.25) divided by the procedure time [3].
En bloc resection was considered when the tumor was resected as a single piece with macroscopic evidence of complete lesion removal. Resection was considered complete (defined as R0) when the tumor was removed en bloc with horizontal (at least 1 mm tumor free) and vertical margins tumor free. Resection was considered incomplete when tumors were removed in fragments (piece-meal), horizontal margins were positive (less than 1 mm tumor free), or margins could not be evaluated due to artificial burn effect (RX). When the vertical margin was positive for carcinoma, resection was defined as R1 [25].
Resection was considered curative when an en bloc R0 resection of a superficial lesion with histology no more advanced than a well-differentiated adenocarcinoma (G1/G2), <1 mm submuco-sal invasion, and with no lymphovascular invasion was achieved [25].
Learning Curve
For an analysis of the learning curve in lesions with severe fibrosis, the study time was divided into 2 periods: first period with resections 1-10 and second period with resections 11-44.
Adverse Events and Post-ESD Management
Perforation was diagnosed either when the muscle layer was injured, and the peritoneal cavity was observed endoscopically, or when free air was found on a plain abdominal radiograph or computed tomography image. Intraoperative bleeding was considered a major adverse event when it required special measures, such as emergency surgery, intraoperative blood transfusion, or vasopressor therapy, or when it led to the premature termination of the procedure. Intraoperative bleeding was considered a minor adverse event when it changed the procedure plan (e.g., use of hemo-clips) or took ≥5 min to be controlled by endoscopy (without meeting criteria for major bleeding). Delayed bleeding was defined when clinical bleeding signs were observed (rectal bleeding or hemoglobin drop >2 g/L) until 30 days after ESD.
Noncurative resections were discussed in a multidisciplinary team and decision regarding subsequent management was made on a case-by-case basis and according to the patient’s preferences. In curative resections, initially, all patients underwent surveillance endoscopy at 3-6 months after the index treatment, and 12 months after that surveillance if no recurrence was found [25]. However, as new evidence appeared, when a curative resection was observed, patients underwent surveillance endoscopy at 12 months after the index treatment [31]. After piecemeal resection or with presence of positive lateral margins without indication for surgery, colonoscopy was performed at 3-6 months [25]. In endoscopic surveillance, a residual disease was defined as the presence of a lesion at the same place where ESD was performed following a non-R0 resection, found in the first or second endoscop-ic control; after this period or if resection was R0, it was considered local recurrence.
Statistical Analysis
Categorical variables are presented as frequencies and per-centages, and continuous variables as means and standard deviations, or median and interquartile ranges for skewed distributions. Continuous parameters were analyzed using Student’s t test or the Mann-Whitney U test, whereas categorical variables were compared using the χ2 test and Fisher’s exact test, as appropriate. Multivariable logistic regression analysis was performed to assess factors predicting severe fibrosis. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated for each variable. Variables were included on the multivariable model and retained in the final model if the p value was <0.1 on univariate analysis.
All reported p values are two-tailed, with a p value of 0.05 indicating statistical significance. Analyses were performed with the use of SPSS software, version 23 (IBM Corp.; Armonk, NY, USA).
Results
Patients and Lesion Characteristics
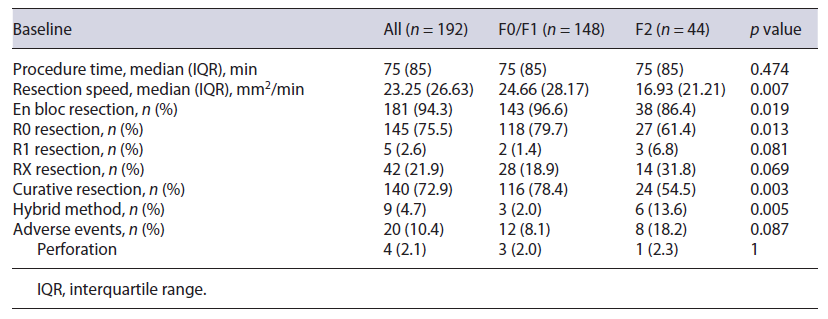
A total of 195 lesions from 192 patients were evaluated (mean age 68.0 ± 11.0 years; 63.6% male). Median tumor size was 40 mm(IQR 27), and lesions were mainly located on the distal rectum (n = 99, 50.8%). Granular-type LST was the most common macroscopic type (n = 140, 71.8%; nodular mixed granular type n = 103, homogeneous granular type n = 37), followed by protruding type (n = 34, 17.4%) and nongranular type (n = 21, 10.8%; flat type n = 13, pseudodepressed n = 8). Three patients had 2 lesions that were simultaneously resected by ESD and 1 patient with noncurative ESD resection had a recurrence treated by ESD. Among the 195 lesions, 150 (76.9%) had no fibrosis or mild fibrosis (F0/F1 group), and 45 (23.1%) had severe fibrosis (F2 group). Resection could not be completed in 3 patients, 2 due to deep submucosal invasion diagnosed during ESD, and 1 due to severe fibrosis, and these were not included in the outcomes analysis. Baseline characteristics are shown in Table 1.
Table 1 Baseline characteristics of included patients and lesions and multivariable analysis of predictive factors for severe submucosal fibrosis
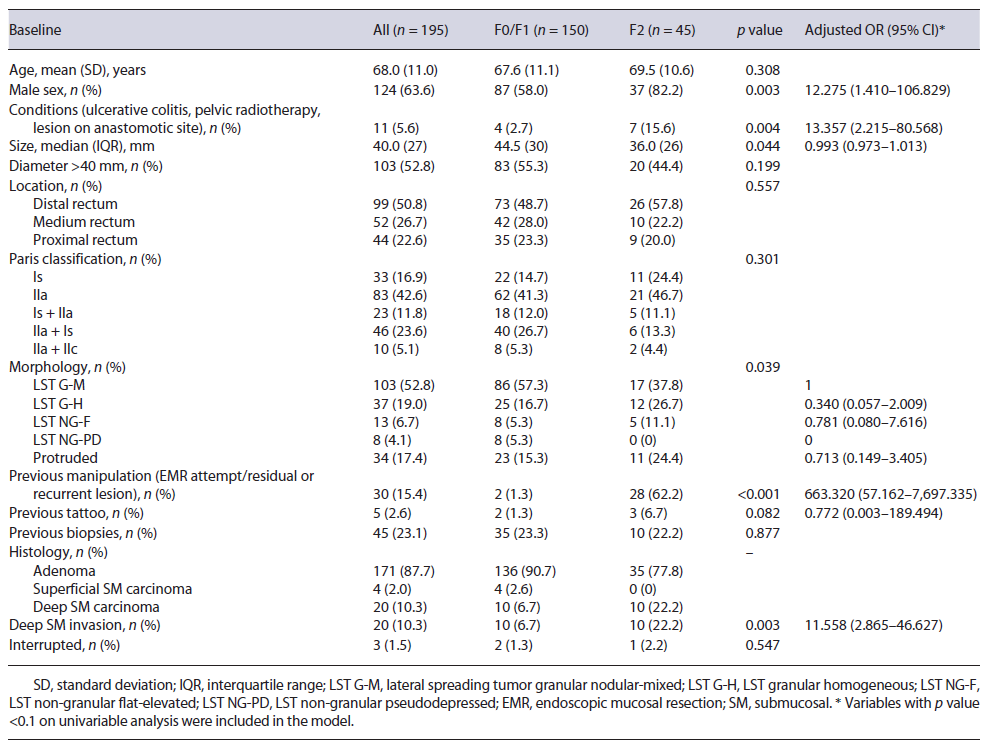
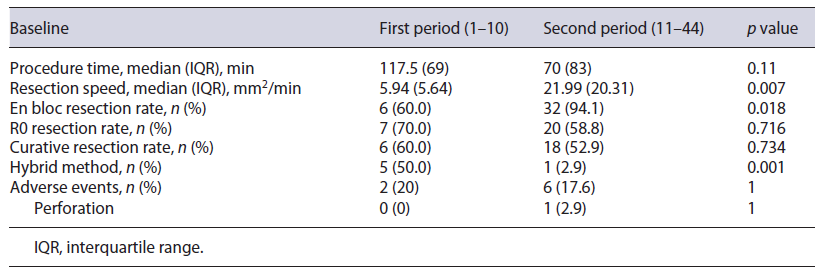
Table 2 shows ESD outcomes of the 192 lesions included (148 in the F0/F1 group and 44 in the F2 group). The median procedure time was 75 min (IQR 85). The overall en bloc, R0, and curative resection rates were 94.3%, 75.5%, and 72.9%, respectively. For tumors considered to have noncurative resections (n = 52), most comprised adenomatous lesions with a positive or no evaluable horizontal margin (n = 34) and 18 had deep submucosal invasion, 5 of which had a positive vertical margin. Hybrid ESD was necessary in 9 (4.7%) resections. Adverse events were observed in 20 patients (10.4%) and included intraoperative minor bleeding in 9 procedures (4.6%), delayed bleeding in 7 procedures (3.6%), and perforation in 3 procedures (1.6%); 1 patient had the procedure complicated by both intraoperative minor bleeding and perforation (0.5%). No adverse event required surgery or discontinuation of the ESD procedure. Additional surgery with lymphadenectomy was performed in 3 patients who underwent noncurative resections; none of these patients had lymph node metastasis.
Severity of Submucosal Fibrosis, Lesion Characteristics, and Procedure Results
Compared to lesions with nonsevere fibrosis, lesions with severe fibrosis were significantly smaller (F2 group: mean size 36 mm, IQR 26; F0/F1 group: mean size 44.5 mm, IQR 30; p = 0.044) and were more frequently associated with deep submucosal invasion (22.2% vs. 6.7%, p = 0.003) (Table 1). There was a significant difference between groups concerning a predisposition for severe fibrosis in the presence of previous conditions (ulcerative colitis, pelvic radiotherapy, or a lesion on an anastomotic site) (15.6% vs. 2.7%, p = 0.004) and previous manipulation, such as a previous EMR attempt or a residual or recurrent lesion (62.2% vs. 1.3%, p < 0.001). Regarding morphology, most lesions in the F2 group were LST G-M (37.8%), followed by LST G-H (26.7%) and protruded lesions (24.4%). No significant difference was found between the two groups regarding patient age, tumor location, previous tattooing, or pretreatment biopsies. The discontinuation rate was 2.2% in the F2 group and 1.3% in the F0/F1 group (p = 0.547).
No difference was observed regarding total procedure time between the F0/F1 and F2 groups (75 vs. 75 min, p = 0.474); however, for lesions in the F2 group, resection speed was lower (16.93 mm2/min vs. 24.66 mm2/min, p = 0.007) (Table 2). Severe fibrosis was associated with significantly lower rates of en bloc (86.4% vs. 96.6%, p = 0.019), R0 (61.4% vs. 79.7%, p = 0.013), and curative (54.5% vs. 78.4%, p = 0.003) resection. A significantly higher rate of hybrid ESD was required to complete resection in the F2 group (13.6% vs. 2.0%, p = 0.005). Adverse events were more commonly observed in the F2 group (18.2% vs. 8.1%, p = 0.087), but this did not reach statistical significance.
Predictive Factors of Severe Fibrosis
Multiple logistic regression analysis was performed to identify independent factors predictive of severe fibrosis. Male sex (OR 12.275; 95% CI 1.410-106.829), the presence of previous conditions (ulcerative colitis, pelvic radiotherapy, or a lesion on the anastomotic site) (OR 13.357; 95% CI 2.215-80.568), previous EMR attempt or residual/recurrent lesions (OR 663.320; 95% CI 57.162-7,697.335), and deep submucosal invasion (OR 11.558; 95% CI 2.865-46.627) were identified as independent predictors of severe fibrosis (Table 1).
Learning Curve of Lesions with Severe Fibrosis Based on the analysis according to earlier and later periods (10 and 34 lesions, respectively), procedure time mproved (earlier period, median 117.5 min; later period, median 70 min; p = 0.11) and resection speed improved significantly (earlier period, median 5.94 mm2/min; later period, median 21.99 mm2/min; p = 0.007). En bloc resection rate was significantly higher in the later period (earlier vs. later, 60.0% vs. 94.1%, p = 0.018), and R0 and curative resection rates were nonsignificantly lower in the later period (earlier vs. later, 70.0% vs. 58.8%, p = 0.716, and 60.0% vs. 52.9%, p = 0.734, respectively). A significantly lower rate of hybrid ESD was required to complete resection in the later period (earlier vs. later, 50.0% vs. 2.9%, p = 0.001). Adverse events were reduced nonsig-nificantly over time (earlier vs. later, 20.0% vs. 17.6%, p = 1) (Table 3).
Long-Term Prognosis after Noncurative ESD
No residual disease nor local recurrence was found in patients with curative resections. Among the 52 patients with noncurative resections, 34 comprised adenomatous lesions with a positive or no evaluable horizontal margin, and during a mean follow-up of 27.3 months, 2 local recurrences were observed and treated endoscopically. The remaining 18 patients had lesions with deep submucosal invasion, and 3 were submitted to salvage surgery with lymphadenopathy (specimen analysis revealed no residual tumor and no lymph node metastasis), 6 were submitted to adjuvant therapy with radiotherapy (with or without chemotherapy), and 9 patients were kept on surveillance; during a mean follow-up of 20.8 months, 1 recurrence was diagnosed and endoscopically treated.
Discussion
In this retrospective study, we aimed to determine whether the presence of severe fibrosis would interfere with the clinical outcomes of rectal ESD. Results of our study demonstrated that the presence of severe fibrosis in submucosa is associated with a lower resection speed (16.93 mm2/min vs. 24.66 mm2/min), lower rates of en bloc (86.4% vs. 96.6%), R0 (61.4% vs. 79.7%), and curative resections (54.5% vs. 78.4%), and higher rate of hybrid ESD required to complete resection (13.6% vs. 2.0%). In our study, no difference was observed in procedure time (75 min), probably because lesions with severe fibrosis were smaller than those without severe fibrosis. Overall, our R0 and curative resection rates were low (75.5% and 72.9%, respectively), especially when compared to our high en bloc resection rate (94.3%). This may be explained by our conservative definition of R0 resection, in which we only considered R0 in the presence of a horizontal margin of at least 1 mm tumor free, contrarily to definitions presented in other studies where only a lateral margin free of tumor was needed to be considered a complete resection [2]. Consequently, despite our low R0 and curative resection rates in lesions with severe fibrosis, only 1 patient developed local recurrence in the nonmalignant group, which was manageable endoscopically; simultaneously, in the noncurative group, due to deep submucosal invasion, only 1 patient presented with local recurrence, which was once again treated endoscopically. We observed a higher rate of adverse events in the excision of lesions with severe fibrosis (18.2% vs. 8.1%); however, all adverse events were minor and were solved conservatively or endoscopically.
Our results reinforce the necessity of accurately predicting fibrosis prior to rectal ESD, as this would allow a more experienced endoscopist to be assigned to more difficult cases, allowing safer procedures. Simultaneously, it would allow some considerations to be taken during the procedure, such as the performance of the initial mucosal incision further away from the lesion than usual, the exposure in advance of the fibrotic areas by thoroughly dis-secting the surrounding nonfibrotic submucosa, the utilization of different settings on the electrosurgical generator unit, and the use of traction methods or alternative strategies to assist the dissection. In a retrospective study, Yoshida et al. [32] described the utilization of the pocket-creation method as an aid for lesions with severe fibrosis, allowing a safer and accurate dissection of the fibrosis and resulting in higher en bloc resection rates, shortened procedure time, and reduced discontinuation rate. Several studies have tried to preoperatively predict fibrosis, mainly based on the morphology of the lesion; however, their results are inconsistent. Matsumoto et al. [8] reported an incidence of F2 fibrosis in LST G-M that was significantly higher than that in LST G-H, Chiba et al. [14] found LST NG-PD to be an independent predictor of fibrosis, and Kaosombatwattana et al. [20] observed that tumors with protruding morphology carried a higher possibility of severe fibrosis. In our study, no morphological aspects were associated with the presence of severe fibrosis, possibly related to the small number of cases. Some studies have reported the influence of preoperative biopsy for rectal lesions on ESD. Fukunaga et al. [13] reported that preoperative biopsies tended to cause F2 fibrosis in the submucosal layer. In our study, no difference was found between the two groups (F0/F1 and F2) regarding preoperative biopsies. However, it should be mentioned that most lesions were referred from other institutions to ESD in our center, hampering the correct assessment of previous biopsies. Makino et al. [33] tried to propose endoscopic ultrasonography as a means to preoperatively assess submucosal fibrosis; however, endoscopic ultrasonography in LST showed only moderate sensitivity and low specificity (77.8% and 57.1%, respectively) for the prediction of fibrosis; as a result, its relevance is still undefined. In our study, severe submucosal fibrosis was independently associated with male sex, the presence of previous conditions, such as ulcerative colitis, pelvic radiotherapy, or a lesion located on an anastomotic site, previous manipulation (EMR attempt and residual or recurrent lesion), and deep submucosal invasion. Nevertheless, our confidence interval was large, due to the small sample size of our study, and caution should be taken when interpreting the results.
We analyzed our learning curve during ESD performed in lesions with severe fibrosis. As expected, procedure time and hybrid ESD decreased, and procedure speed and en bloc resection rate increased over time.
Contrary to our predictions, a nonsignificant reduction in R0 and curative resection rates over time was noted. This may be explained by an increase in the complexity of lesions removed by ESD in our center over time. As our experience increased, more complex cases were referred. As an example, we performed ESD for coalescent polyps located in the anal transitional zone after restorative proctocolectomy and ileal-pouch anal anastomosis in a patient with familiar adenomatous polyposis [34].
Our study has several limitations. First, it is a single-center retrospective study and is limited by a small sample size, especially for subgroup analysis. Second, we evaluated the degree of fibrosis based on endoscopic findings during the procedure. Histopathological assessment may be more objective than clinical assessment, which depends on the judgment of the operator; however, many studies reported clinical evaluation of the degree of fibrosis by the endoscopist during the procedure [8, 10-13, 19]. Histological assessment may be influenced by the dissection procedure, the depth of the dissection, or thermal injury by electrocoagulation [13]. Therefore, histologic severity of fibrosis does not necessarily reflect endoscopic severity, and subsequently its clinical relevance. Third, we have a mean follow-up of about 2 years, which might limit the evaluation of recurrence, particularly metastatic disease. A main strength of our study was the performance of all the procedures by the same endoscopist, circumventing performance variation between different endoscopists. However, caution should be taken when extrapolating the results.
In conclusion, ESD is a safe and effective treatment for complete resection of lesions with severe fibrosis, despite being associated with lower en bloc, R0, and curative resection rates, even with expert endoscopists. The presence of ulcerative colitis, pelvic radiotherapy, a lesion located on an anastomotic site, or previous manipulation can preoperatively help to predict cases with severe fibrosis. To the best of our knowledge, this is the first European study to assess the relevance of severe fibrosis on the ESD outcomes and the learning curve of lesions with severe fibrosis.