Introduction
Primary colorectal lymphoma is a very rare condition, accounting for 0.2% of all colorectal malignancies [1-3]. Its association with ulcerative colitis (UC) is mainly related to the use of immunomodulators, which dictate a definite increase in risk of lymphoproliferative disorders (LDs). In the absence of immunosuppressive therapy, the risk of LD in inflammatory bowel disease (IBD) patients seems to be similar to that of the general population [4]. Here in we describe a case of a particularly rare form of diffuse large B-cell lymphoma (DLBCL), with rectal involvement, in a patient with ulcerative proctitis with no history of immunomodulator exposure. The disease presentation simulated an acute flare of the patient’s chronic disease.
Case Report
We present the case of a 42-year-old male with a 7-year history of ulcerative proctitis treated with topical mesalamine (suppository 1 g/day) and no previous exposure to immunomodulatory agents or corticosteroids. His medical history was uneventful. He had a family history of IBD but no history of neoplasia or LD. In the last colonoscopy, performed in March 2019, the patient was in endoscopic remission (Mayo subscore 0).
In September 2020 the patient developed diarrhoea with 3-4 bowel movements per day, the stools consisting of watery fluids and mucus, without blood. Three months later, symptoms per-sisted despite intensive topical and oral treatment with mesalamine (suppository 1 g/day + oral mesalamine 4.5 g/day). At this time, the patient also complained of severe rectal pain, tenesmus and weight loss (10% in 2 months). He denied fever, fatigue or night sweats. Topical budesonide was prescribed without improvement. Oral prednisolone was then started, and colonoscopy performed, revealing a suspicious rectal lesion. The patient was admitted to the hospital to proceed with the investigation and for pain management.
At admission, the patient complained of severe rectal pain, only controlled with tramadol perfusion. On physical examination, the patient was haemodynamically stable and had no fever. There were no enlarged palpable lymph nodes. Abdominal examination did not show any signs of tenderness or palpable masses, but on rectal examination, a palpable firm mass was detected. Blood tests revealed a slightly elevated C-reactive protein (1.17 mg/dL) with normal white blood cell count (10.9 × 109/L). Abdominal X-ray was normal. An urgent computed tomography (CT) revealed the presence of a circumferential lesion located at the lower/mid rectum with associated wall thickening. Significant luminal narrowing was also described (Fig. 1). A pelvic magnetic resonance imaging (MRI) was further conducted, showing a concentric circumferential rectal wall thickening with ulceration beginning at 3 cm from the anal verge and extending longitudinally along 12 cm. This mass invaded the mesorectal fascia and the right sphincter complex, having a defined cleavage plane with the prostate and bladder. Regional lymph node involvement was observed (Fig. 2).
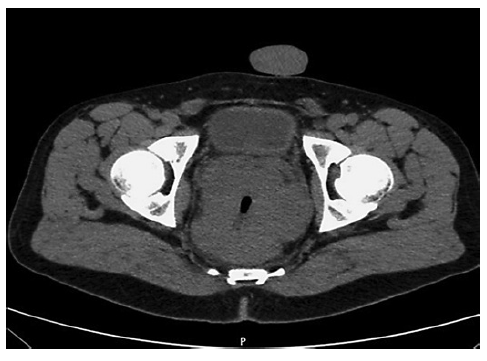
Fig. 1 Abdominal CT scan showing circumferential stricturing lesion located at the lower/mid rectum with associated wall thickening.
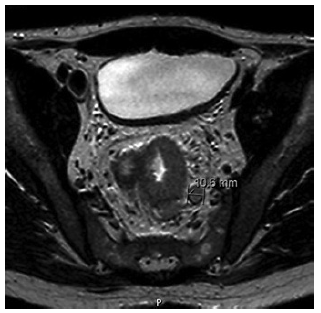
Fig. 2 Pelvic MRI confirmed the presence of rectal wall thickening with ulceration extending from 3 to 12 cm above the anal verge. Regional lymph node involvement was also observed.
Complementary laboratory studies were obtained; namely, tests for sexually transmitted diseases, including human immunodeficiency virus (HIV) types 1 and 2, herpes simplex virus (HSV) type 2, Chlamydia trachomatis and Treponema pallidum, were negative; serology for HSV type 1 and Cytomegalovirus revealed the presence of specific IgG antibodies; serological marker for hepatitis C virus (HCV) was negative and hepatitis B surface antibody was detected for hepatitis B virus (HBV). Evaluation of the Epstein-Barr virus (EBV)-specific antibody profile revealed positivity for IgG with equivocal IgM. The viral load measurement was further added to the study, confirming the presence of an acute infection (5,972 copies/mL).
A lower gastrointestinal (GI) endoscopy was performed, revealing a circumferential lesion extending continuously from 12 cm above the anal verge to the dentate line, with associated stenosis. The lesion was extensively ulcerated with some areas covered with grey necrotic tissue (Fig. 3). The histopathological analysis of the rectal biopsies revealed lymphocyte infiltration in all layers of the rectal wall (Fig. 4a). In the immunohistochemical staining, cells were immunoreactive for CD20, CD10, bcl-6 and c-myc; the Ki67 immunohistochemical stain depicted 100% proliferative index (Fig. 4b). Detection of EBV by in situ hybridization to EBV-encoded RNA was also positive (Fig. 4c). Fluorescence in situ hybridization analysis revealed positive C-MYC gene rearrangement in 80% of analysed nuclei. Based on these findings, the diagnosis of a high-grade non-Hodgkin lymphoma (NHL) - DLBCL type was considered. After a lymphoma-negative bone marrow biopsy infiltration and negative neck and thorax CT scan, needed to complete adequate staging, the final diagnosis of primary rectal NHL DLBCL stage IV was established, and chemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) and high-dose methotrexate was started. Colonoscopy evaluation after 6 cycles of chemotherapy revealed complete endoscopic remission (Fig. 5). In the last follow-up visit (November 2021), 4 months after treatment, the patient remains in complete remission, for both the NHL DLBCL and UC.
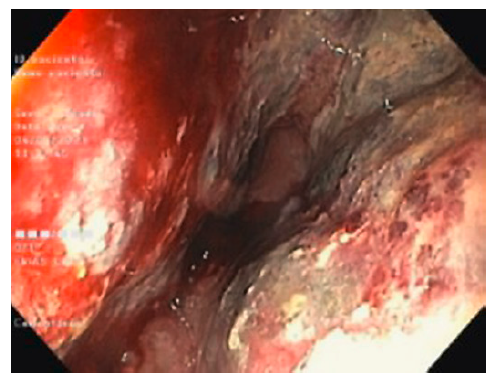
Fig. 3 Colonoscopy showing circumferential and extensively ulcerated lesion of the rectum, with associated stenosis.
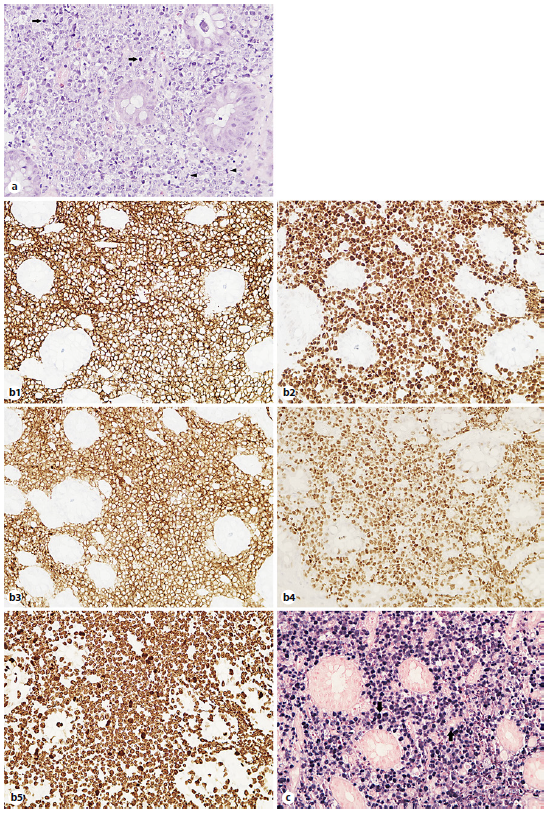
Fig. 4 Rectal biopsies. a Infiltration of the rectal wall by lymphoid cells, where mitotic figures (arrowheads) and apoptotic cells (arrows) are easily recognized. Haematoxylin and eosin stain. Magnification ×400. b1-5 Immunohistochemical examination: atypical lymphocytes are CD20, CD10, bcl-6 and c-myc positive with Ki67 immunohistochemical stain depicting 100% proliferative index. c Detection of EBV by in situ hybridization to EBV-encoded RNA was positive (blue staining) in the tumor cell nuclei (arrows). Magnification ×400.
Discussion/Conclusion
The GI tract is the major site of extranodal NHL and accounts for 30-40% of all cases [2]. While secondary GI involvement is relatively common, primary GI lymphomas are rare, representing only 1-4% of malignancies arising in the GI tract [3, 5]. They are generally defined as lymphomas that predominantly involve any section of the GI tract, in the absence of evidence of systemic disease [6]. They have unique clinical aspects and management considerations [2].
The most common site of primary GI lymphomas is the stomach (50-60%), followed by the small intestine (20-30%) [5, 7]. Involvement of the colon is rare (10-20%), with the cecum being the segment most commonly affected. Primary rectal lymphoma is the rarest location of lymphomas, accounting for 0.05% of all primary rectal malignancies [8].
Primary colorectal lymphomas have a male predominance, with men being twice more often affected than women. It usually occurs in the sixth to seventh decade and presents with abdominal pain, weight loss or changing in bowel habits [2, 3]. Less frequently described symptoms include lower GI bleeding (20% of patients) and obstruction, which occurs less often than in colorectal adenocarcinoma [3, 7].
Among the different histological subtypes of primary colorectal lymphomas, DLBCL is the most common [5, 7, 9], being generally aggressive. Thus, prognosis is poor with a 5-year survival rate of 27-33% [10].
Specific risk factors for primary colorectal lymphomas are largely unknown. However, it is generally assumed that the established risk factors for NHL will also increase the risk for primary colorectal lymphoma. Viral infections (namely HIV, HCV, HBV and EBV), immunosuppressive medications, organ transplantation, family history of lymphoma and personal history of chemo- and/orradiotherapy are the main risk factors described in the literature [2]. Some chronic systemic diseases such as autoimmune and immunodeficiency disorders are intrinsically associated with an increased risk of LDs [2, 4]. Contrarily, according to some studies, IBD on its own does not seem to increase the risk for primary colorectal lymphoma [4, 11]. However, when IBD patients are exposed to immunosuppressive therapy, the risk increases significantly. Thiopurines and, to a lesser extent, anti-tumour necrosis factor agents are the drugs associated with excess risk of lymphomas [11]. This LD is often EBV-associated. While the virus promotes malignant transformation, immunosuppressive therapy inhibits the immune system´s ability to detect and clear malignant cells [4]. In fact, in the CESAME cohort, the risk of LD was highest in those exposed to thiopurines, with a four- to sixfold increase in risk, and 46% of cases were EBV positive [4, 11].
In the present case, however, primary rectal DLBCL developed in the absence of previous or current immunomodulatory therapy for the treatment of UC. Although being extremely rare, similar cases have been reported in the literature [10, 12, 13], raising the hypothesis of alternative mechanisms contributing to the development of DLBCL in UC patients [10]. One of the possible mechanisms is overstimulation of the immune system secondary to chronic colorectal inflammation. In fact, previous studies have suggested that extensive or long-standing IBD seems to be associated with the development of lymphomas in IBD patients, with the mean time between the diagnosis of UC and lymphoma being 12 years [10, 14]. Moreover, lymphoma usually develops in areas affected by intense inflammation.
The underlying possible mechanism is chronic inflammation leading to clonal lymphocyte activation and sub-sequent proliferation. The maintained lymphocyte stimulation increases the possibility of mutations with consequent development of malignant lymphoma [14]. Additionally, EBV infection may also play a role in the development of lymphoma in long-standing IBD pa-tients, even in the absence of immunosuppressive therapy. Thus, chronic local inflammation may induce a localized immunodepression, favouring the clonal proliferation of EBV-infected B cells [4, 15].
In our case, although the DLBCL developed in a time interval (7 years) that was shorter than that usually reported in the literature, chronic local inflammation may have triggered clonal proliferation of EBV-positive lymphocytes. Although the previous status of EBV infection of our patient is not known, the most probable scenario is the reactivation of a latent EBV infection. Critical association of EBV-associated lymphoproliferative diseases with viral latency and contribution of specific latent antigens to B-cell transformation has been described [16].
In the present case, the clinical presentation mimicking an acute flare of UC and the absence of rectal mucosal lesions in the endoscopic evaluation performed 1 year before posed a diagnostic challenge, highlighting the complexity behind the management of UC patients. This case report emphasizes that, although being rare, the occurrence of colorectal lymphoma should be taken into consideration in UC patients presenting with refractory disease, even when there is no history of previous exposure to immunomodulatory agents, to ensure proper diagnosis and treatment.