Introduction
Colorectal cancer (CRC) is a leading cause of morbidity and mortality in the world, especially in Western countries [1, 2]. Worldwide, CRC accounts for 860,000 deaths [2]. Colonoscopy has been shown to decrease both the incidence of CRC and the related mortality by facilitating the detection and allowing the removal of adenomas [3-8] and is endorsed as the preferred option for CRC screening and adenoma surveillance [9-12]. The adenoma detection rate (ADR) is currently the main quality indicator for colonoscopy [13, 14] as a higher ADR results in lower risk of CRC and mortality [15]. However, conventional colonoscopy has been shown to miss lesions in tandem studies, especially sessile serrated lesions (SSLs) [16-18]. These lesions are different from adenomas; they are more frequent on the right colon and usually present with a flat morphology that makes them much harder to detect through optical colonoscopy. SSL also presents a different, faster carcinogenesis pathway and as result of these characteristics, they are associated with interval CRC, which is the occurrence of CRC after screening colonoscopy and before the next scheduled screening procedure [19, 20].
Narrow band imaging (NBI) has been shown to be effective for SSL detection in one trial performed in an academic center and in the setting of sessile serrated polyposis [21, 22]. In another RCT, Rex et al. [23] compared NBI (OlympusTM 190 series colonoscopes) and high-definition white light (HD-WL) colonoscopy for the detection of proximal serrated lesions in average-risk individuals. This trial showed a trend toward higher detection in the NBI but failed to achieve statistical significance for the primary endpoint (number of proximal serrated lesions) [23]. Few other trials have studied the effect of NBI on the detection of colorectal polyps and adenomas and some have also reported the incidence of serrated class lesions with nonsignificant results in most of them [24-27]. Recently, a meta-analysis pooled the results of these trials which showed a significant increase in the detection of serrated lesions with NBI [28].
Therefore, it is still unsettled whether NBI should be used systematically during colonoscopy withdrawal to increase detection of CRC precursor lesions. Our aim was to evaluate if the systematic usage of NBI during colonoscopy withdrawal contributes to a higher rate of SSL detection in an average CRC risk population.
Materials and Methods
Study Design
We performed a 2-arm superiority RCT to compare SSL detection between NBI and HD-WL optical colonoscopy. The study was approved by the Institutional Review Board at Hospital Beatriz Ângelo and NOVA Medical School and was registered at clinical-trials.gov (NCT02876133). Patients were required to sign a written informed consent. The study was performed in one academic center between October 2016 and February 2021.
Study Population
Consenting individuals fulfilling the inclusion criteria were patients scheduled for elective colonoscopies, aged 40-74, cecal intubation and adequate bowel preparation according to the Boston Bowel Preparation Score (BBPS) >1 in each bowel segment, and without exclusion criteria: known polyposis syndromes, primary sclerosing cholangitis, inflammatory bowel disease, personal CRC history or colorectal surgery, contraindications to polypectomy, current pregnancy, and ASA > 3.
Outcomes
The primary endpoint was the average number of serrated lesions including hyperplastic lesions ≥10 mm detected per colonoscopy. The secondary endpoints were SSL detection rate (number of patients with at least 1 SSL/total number of participants); serrated class lesions detected per colonoscopy (number of serrated lesions/total number of participants); ADR (number of patients with at least 1 adenoma/total number of participants); adenomas detected per colonoscopy (number of adenomas/total number of participants); malignant adenocarcinoma detection rate (number of malignant adenocarcinomas/total number of participants); incidence of procedure-related adverse events; withdrawal time.
Study Procedures and Data Collection
We used a block randomization table generated in STATA which was uploaded to the eCRF software and not accessible to the investigators. Randomization was concealed before the procedure and until patient assignment which occurred only after cecal intubation using the REDCap platform. Consenting patients were randomized to the NBI group or the white light colonoscopy group, after cecal intubation and before the withdrawal. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Sociedade Portuguesa de Gastrenterologia [29, 30]. REDCap is a secure, Web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails to track data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures to support data integration and interoperability with external sources.
The six participating endoscopists were all experienced in optical colonoscopy (defined by having performed a minimum of 300 colonoscopies) [31] and electronic chromoendoscopy with an ADR above 40% in all cases. The procedures were performed using a high-definition Olympus endoscope (CF-H190 or GIF-H190). Colonoscopies were performed either without sedation, under conscious sedation or under deep sedation, as requested by the assistant physician. Antispasmodics (butylscopolamine) could be administered during the procedure at the endoscopist discretion.
The histologic evaluation of each lesion was performed by pathologists in our center. The pathologists were blinded to the method used during the procedure. We recorded patient demographic and clinical data, including date of birth, sex, weight, height, body mass index, education level, smoking habits, personal history of polyps and polypectomy, date of previous colonoscopy, and family history of CRC; colonoscopy data, such as the endoscopist performing the procedure, colonoscope model, indication for the procedure, depth of sedation (no sedation, conscious, or deep sedation), the administration of antispasmodics (butylscopolamine), intubation and withdrawal times, Boston Bowel Preparation Score (BBPS) in each colon segment (ascending, transverse, and left colon), and adverse events; and for each lesion detected the location, size, morphology (Paris Classification [32]), and histology (hyperplastic, adenoma, SSL, or adenocarcinoma).
Sample Size
The prevalence of SSL at screening colonoscopy is close to 5% but ranges from 1 to 18%, with a mean of 1.62 lesions per case [33, 34]. For serrated lesions ≥10 mm, we based our estimate on Rex’s trial [23] which had a proportion of 0.098 proximal lesions per colonoscopy with NBI. We believed that a 100% increase in yield could be a sufficient difference to consider routine use of NBI. Therefore, considering the number of lesions per patient as the primary endpoint and to have an 80% power at a 5% significance level to detect a difference from 0.049 to 0.098 lesions/colonoscopy, we would need a total sample size of 968 colonoscopies. We anticipated a 2% cross-over rate and therefore we adjusted the sample size to 987 colonoscopies. Cross-over was anticipated to occur in case of poor judgment of the bowel preparation quality where white light would be needed instead of NBI and in case of error by the endoscopist or equipment malfunction.
The statistical analysis was conducted with the SPSS software package, version 21 (Statistical Package for the Social Sciences; IBM Corporation, Armonk, NY, USA). Categorical variables are expressed as frequencies and percentages, while continuous variables are described as the means and standard deviations or medians and ranges. The χ2 test and Fisher’s exact test were used to explore associations between categorical variables. Differences in means for continuous variables and dichotomous variables were analyzed by t tests or Mann-Whitney U tests, as appropriate. The study was prematurely terminated due to the significant impact of COVID-19 pandemic on recruitment pace.
Results
Patient and Procedural Characteristics
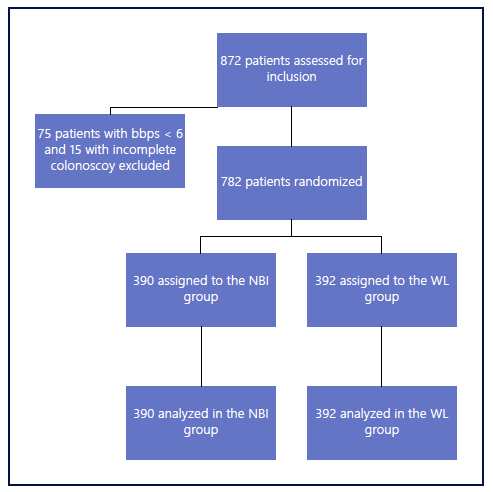
A total of 872 patients were assessed for eligibility, with 90 patients excluded before randomization due to poor bowel preparation (n = 75) and failure to reach the cecum (n = 15). From the included 782 patients, 390 were randomly assigned to NBI and 392 to HD-WL group. All patients received the allocated intervention. The trial profile is depicted in Figure 1.
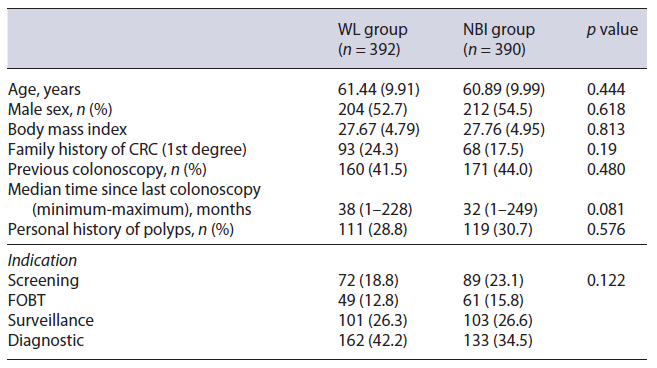
Table 1 summarizes baseline characteristics. There were no differences between the two study groups regarding age, sex, family history of CRC, personal history of polyps, and colonoscopy indication.
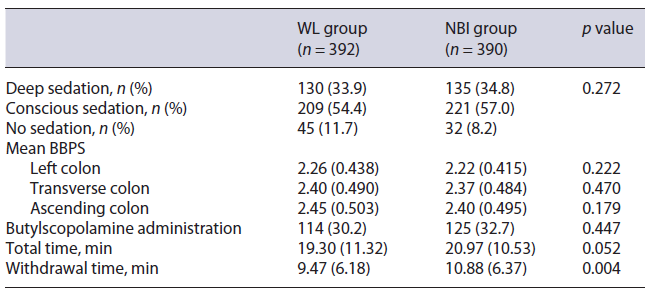
Table 2 shows procedural characteristics. Mean withdrawal time was 1.41 min higher in the NBI group (10.88 vs. 9.47 min, p = 0.004), with a statistically non-significant higher total procedure time (20.97 vs. 19.30 min, p = 0.052). No significant differences were observed between the two study groups regarding depth of sedation, administration of antispasmodics (butylscopolamine), and bowel preparation quality in each colonic segment.
Outcomes
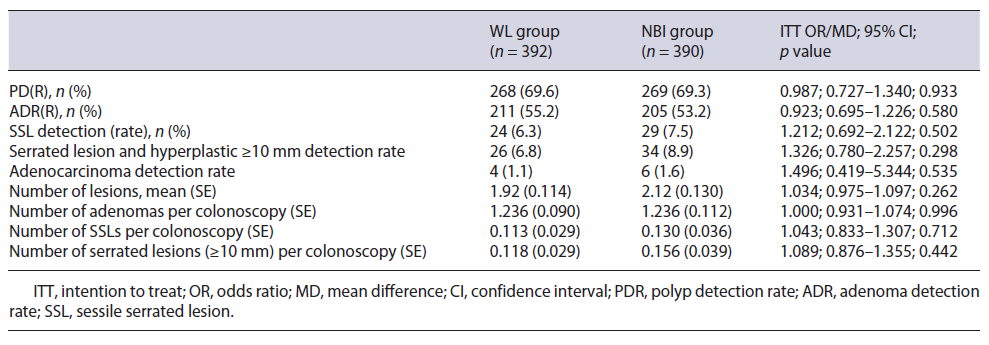
Table 3 summarizes the proportion of detected lesions by study group (HD-WL vs. NBI group). For the primary endpoint of the average number of serrated lesions and hyperplastic polyps ≥10 mm detected per colonoscopy, there was no significant difference between the two groups (0.118 vs. 0.156, p = 0.44).
Overall, no differences were observed in polyp detection rate (69.6% vs. 69.3%, p = 0.93), ADR (55.2% vs. 53.2%, p = 0.58), SSL detection rate (6.3% vs. 7.5%, p = 0.502), and serrated lesions including hyperplastic ≥10 mm detection rate (6.8% vs. 8.9%, p = 0.298) between HD-WL and NBI groups. Likewise, the number of adenomas (1.23 vs. 1.23, p = 0.996) and SSLs (0.11 vs. 0.13, p = 0.712) per colonoscopy was also not different. Finally, the adenocarcinoma detection rate was also similar (1.6% vs. 1.1%, p = 0.535).
Discussion
We performed a randomized controlled trial design to determine whether NBI improves the detection of serrated lesions and hyperplastic lesions ≥10 mm. Our results did not show a significant difference in the detection of these lesions or in any other lesions (adenomas, SSLs, all polyps, and invasive cancer). It is important to acknowledge the high detection rates (ADR of 54% and SSLR of 7%) in this study as the effect of optimization strategies decreases with high detection rates.
Nonetheless, our study is in line with the large RCT performed by Rex et al. [23] which recruited 800 patients and looked at the detection of serrated class lesions proximal to the sigmoid colon and only found a nonsignificant trend in favor of NBI (204 vs. 158, p = 0.085) [23]. However, in a recent meta-analysis, which included three studies and pooled the results of 1931 colonoscopies, there was a higher detection of serrated adenomas in the NBI group (RR 2.04, 95% confidence interval: 1.18-3.54, p = 0.001) [28]. Yet, none of the included trials was specifically designed for serrated lesions and only Visovan et al. [24] reported a positive result. This was the trial with the highest weight in the meta-analysis, but it did not actually report the SSLs detection in the original manuscript published in the Bosnian Journal of Basic Medical Sciences [24]. Another relevant limitation of the meta-analysis is the exclusion of the 800 patients’ trial by Douglas Rex because it used proximal serrated lesions as the end-point instead of histologically determined SSLs.
Another important point of our study was the increased inspection (withdrawal) time by an average of 85 s in the NBI group. We believe this effect was probably associated with the known need for better washing and suction of the colon as NBI image is severely impaired by the presence of colonic residue and even clear fluids. This effect has also been seen in other trials studying NBI [28].
Strengths of this study include the randomized design and large sample size, using an endpoint that included SSLs according to the pathologist, and large hyperplastic lesions which are also a significant finding. An option would be to have all endoscopically suspicious lesions for serrated morphology double checked by a second expert digestive pathologist.
The main limitations were the uncontrolled withdrawal time which was higher in the NBI group and the impossibility to blind the endoscopist, which is inevitable in these studies. However, we have previously studied and reported colonoscopy quality outcomes that may help as a benchmark. Previously, we published in GE an observational study from 2012 to 2014 with a routine ADR of 36% and an SSL detection rate of 1% [35]. These figures improved in our latest report with data from 2017 to 2019 with an ADR of 55% and SSL detection rate of 4% [36]. The data shown demonstrate the overall detection improvement during routine colonoscopies in our department in recent years and are in line with the outcomes reported in our control group. Nevertheless, one must acknowledge that the prevalence of pathology is increased by including cases not restricted to a pure screening population. Another important limitation is that our study was prematurely terminated due to COVID-19 pandemic and we were 205 hundred cases short. To better understand, we calculated that this sample with these results has a power of 71% to detect the prespecified effect in the sample size calculation. Therefore, it would be very unlikely that with an extension of the trial the primary end-point would be met.
In this study, we used SSLs and large hyperplastic polyps as a combined endpoint to overcome the limitation of the known pathological identification of SSL. Unlike in Rex’s trial [23], we did not include all proximal hyperplastic lesions, and this may have contributed to a smaller effect of NBI.
In the future, studies should have a large sample size determined by the endoscopists (and pathologists) detection rates and include data on location, size, endoscopic assessment, and histology of all lesions in order to detect small differences and to allow effective meta-statistical assessment of the existing trials. Finally, we must acknowledge that although NBI did not improve the detection of serrated lesions, it has been shown to be useful in other situations such as the characterization of epithelial lesions [37, 38].
The present study is one of the largest randomized controlled trials studying the effect of NBI for the detection of colorectal lesions and more specifically SSLs and large serrated class lesions. It failed to show a significant effect other than an increase in the withdrawal time. We conclude that a beneficial detection effect of NBI is unlikely and overwhelmed by an increase in procedural time.
Acknowledgment
The authors would like to acknowledge the support of the Portuguese Society of Gastroenterology by granting the use of the REDCap software. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Sociedade Portuguesa de Gastrenterologia. REDCap is a secure, Web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.