Introduction
Hypercholesterolemia is a common finding in clinical practice. However, few conditions are associated with total cholesterol (TC) values greater than 1,000 mg/dL, primarily in patients with homozygous familial hypercholesterolemia and family lecithin acyltransferase deficiency [1, 2]. As the liver plays a pivotal role in lipid and lipoprotein metabolism, cholestatic liver diseases are associated with high levels of an abnormal lipoprotein with a density similar to very-low-density lipoprotein and low-density lipoprotein but different composition, the lipo-protein X (LpX) [3]. Recognition of this entity is of ut-most importance as lipid-lowering agents are ineffective, and definitive treatment requires correcting the underlying cause of cholestasis [4].
Case Presentation
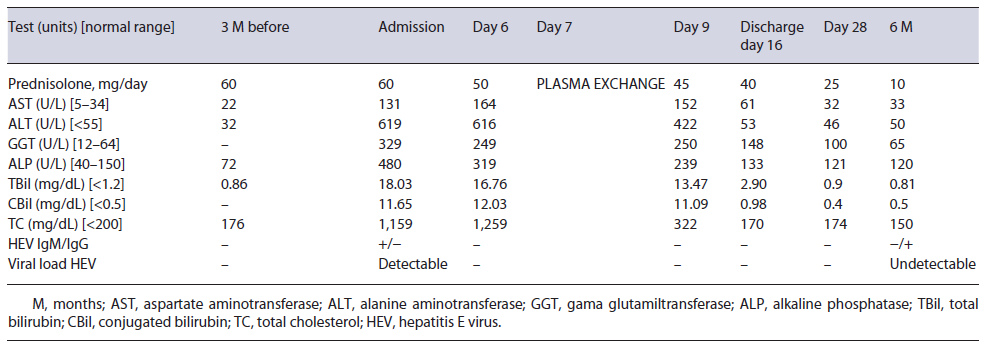
A 44-year-old Caucasian male was admitted to our gastroenterology department due to a 1-week clinical picture of jaundice, choluria, asthenia, and anorexia. He denied fever, abdominal pain, pruritus, weight loss, nausea, and vomiting. His medical history was remarkable for orbital inflammatory pseudotumor treated with prednisolone (60 mg/day) in the last 3 years. He has no family history of early-onset coronary artery disease or hyperlipidemia. Three months prior, his routine workup showed regular lipid panel and liver enzymology (shown in Table 1). There was no relevant epidemiological background, such as recent travel and eating uncooked meat. The patient denied alcohol or drug consumption. The physical exam only revealed jaundice; the remaining examination excluded encephalopathy, ascites, xanthelasma, or xanthomas.
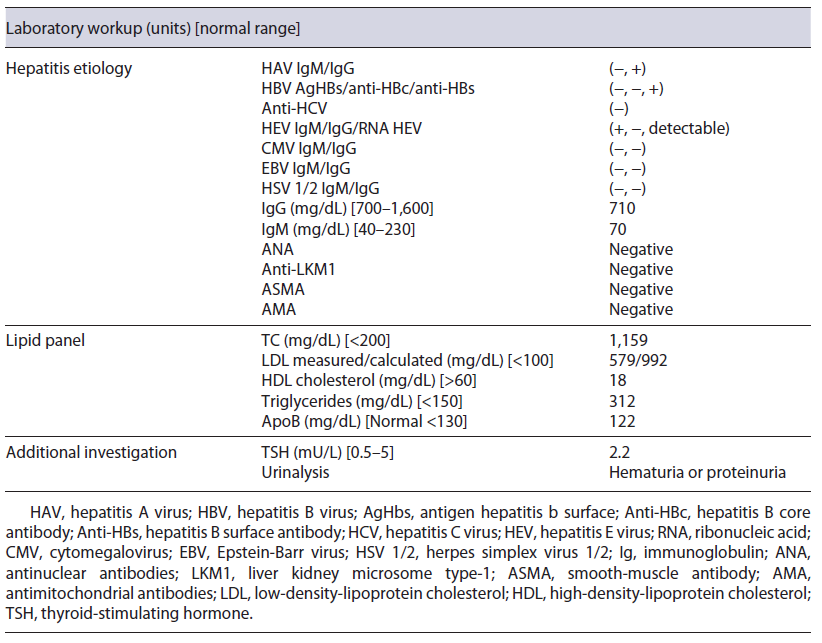
Initial blood investigations revealed elevated hepatic enzymes displaying a mixed pattern (ALT >15 times and ALP > four times the upper limit of the reference range; with R factor 4.5) and severe hypercholesterolemia (TC >1,000 mg/day) (shown in Table 1). Blood counts, albumin, glycemia, and coagulation tests were unremarkable. Abdominal computed tomography excluded biliary obstruction. Moreover, the diagnostic workup showed acute hepatitis E virus (HEV) features, excluding the remaining viral and autoimmune etiologies (shown in Table 2). A percutaneous liver biopsy denoted cholestasis, mixed lobular inflammatory infiltrate, and focal hepatocellular necrosis. We decided to taper prednisolone with close ophthalmologic surveillance; we did not start anti-lipemic medications.
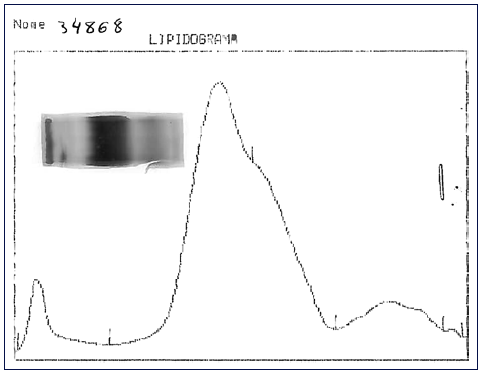
Fig. 1 Lipid electrophoresis: application point corresponds to the fraction with low protein content.
Further investigation of the hyperlipidemia ruled out hypothyroidism and nephrotic syndrome (shown in Table 2), and we excluded primary causes due to the new onset and the absence of family history. We hypothesized hypercholesterolemia secondary to LpX due to cholestatic viral hepatitis. This diagnosis was supported by TC elevation without proportional elevation of apolipoprotein B (ApoB) and a calculated LDL cholesterol higher than measured (shown in Table 2). A nonquantitative fasting lipoprotein electrophoresis showed a band at the application site with slow migration, suggesting the presence of low density and protein fraction, such as Lp-X (Fig. 1).
Given the persistently high lipid levels, which could trigger complications related to hyperviscosity syndrome, the patient underwent plasma exchange, with a marked decrease in plasmatic cholesterol (shown in Table 1), after one single session. Along with the tapering of corticosteroids, we observed a favorable clinical course, with a complete resolution of liver tests (shown in Table 1).
No new ophthalmological alterations were detected, the lipid profile remained normal (without anti-lipemic drugs), and we exclud-ed chronic HEV infection.
Discussion
The liver is a critical organ in lipid and lipoprotein metabolism. In 1969, Seidel et al. [5] described for the first time a lipoprotein rich in phospholipid and free cholesterol in patients with cholestasis; due to its unknown origin, it was denominated as Lp-X. LpX is an abnormal lipoprotein with a density similar to very-low-density lipo-protein and LDL but different lipid and apolipoprotein compositions [3, 5, 6]. It is rich in phospholipids and free cholesterol (80-90%) and poor in cholesterol esters (<5%), triglycerides, and proteins. Unlike LDL, it contains albumin dissolved in its aqueous core. The apolipo-proteins encountered on its surface comprise Apo AI, E, and C, although never ApoB [3, 5, 6].
The pathogenesis is poorly understood. Biologically, cholesterol is eliminated by conversion to bile acids [3]; however, when biliary stasis develops, there is progressive retention of bile salts in hepatocytes, which stops the production of new bile acids accumulating cholesterol within the liver cells [3, 7, 8]. Subsequently, there is a reflux of cholesterol into the circulation [4]. LpX is associated with cholestatic liver diseases, including primary biliary cholangitis [4], bile duct obstruction, graft versus host disease in liver transplant recipients [9], deficiency of lecithin acyltransferase enzyme [1], granulomatous hepatitis [10], and other causes of hepatitis [11].
HEV infection is considered one of the common causes of acute fecal-oral-transmitted hepatitis in developing countries [12]. Still, in developed countries, HEV is estimated to account for less than 1% of cases of acute viral hepatitis despite being an often-overlooked cause. Evidence suggests that autochthonous transmission is zoonotic due to undercooked pigs and deer [13, 14]. HEV has an incubation period of 3-8 weeks, followed by a prodromal phase and acute icteric hepatitis lasting for days to several weeks. Acute HEV is usually clinically silent [13]. Marked cholestatic hepatitis with persistent jaundice and elevation of serum alkaline phosphatase is uncommon. In immunocompromized patients, anti-HEV IgM may be falsely negative; therefore, the gold standard for diagnosing acute HEV is the detection of HEV RNA in biological samples [13]. Acute HEV infection is usually self-limiting in immunocompetent patients and requires only supportive treatment. However, in immunocompromized patients, primarily reported in solid organ transplant recipients [15], HEV may not be cleared spontaneously and causes chronic hepatitis. Reducing the dosage of immunosuppressive therapy helps eliminate HEV [16, 17], so we reduced the immunosuppressive load with clinical improvement and no evolution to chronic HEV. Ribavirin is the treatment of choice in chronic HEV (14).
Cholesterol values greater than 1,000 mg/dL may be encountered with the presence of the LpX fraction [7, 18]. Methods for its direct determination are poorly available in conventional laboratories [18], so knowledge about the composition of this lipoprotein may be helpful in diagnosis. LpX does not contain ApoB, so it is frequent to find an excessive elevation of TC that is not accompanied by a proportional elevation of ApoB (increased TC/ApoB ratio) [4], as in our patient. A discrepancy between LDL-C calculated by the Friedewald formula and measured supports the presence of LpX [3, 4, 18]. In our patient, the calculated LDL cholesterol was 992.8 mg/dL, whereas the measured one was 579 mg/dL; this is because LpX interferes with the precipitation of chylomicrons, preventing their correct measurement. Demonstrating the presence of LpX can be done by lipoprotein electrophoresis using a commercially available kit [4, 18]; a low protein fraction may exist near the application point [18], as we observed in our case patient. Other alternative techniques for LpX determination have also been described, for example, agarose electrophoresis, non-denaturing polyacrylamide gradient gel electrophoresis, and nuclear magnetic resonance spectroscopy [18].
Apo-B’s absence results in LpX uptake by hepatocytes instead of macrophages, so liver dysfunction drives lower clear-up LpX from the plasma. Therefore, correction of the underlying cause is pivotal. Lipid-lowering agents such as statin and ezetimibe will not affect cholesterol levels in this setting and carry potential toxicity [4]. Lipoprotein apheresis is a temporary measure usually reserved for complications such as hyperviscosity syndrome, xanthomata, and cholesteroloma [4, 18, 19] when underlying cause resolution is not feasible or inadequate. The benefit appears to be greater with plasmapheresis than with lipoprotein apheresis [20]. Given that our patient had persistently high cholesterol levels and the high immunosuppressive burden delayed the resolution of the cause of cholestasis (lower viral clearance of HEV), he successfully and safely underwent plasmapheresis.
Thus, considering our case report, in routine practice, abnormalities in the lipid panel should raise suspicion about the presence of LpX, especially in patients with hypercholesterolemia and cholestasis. Recognizing this condition allows the correct therapeutic management and the prevention of complications. We describe the first case reported in the literature of LpX-associated hypercholesterolemia due to acute hepatitis E, responding to spontaneously eliminating HEV after weaning immu-nosuppressive therapy, aided by concomitant plasmapheresis (one session).