Introduction
Gastrointestinal (GI) fistulas are one of the most feared adverse events after abdominal surgery and constitute the second-leading cause of death among patients undergoing bariatric procedures, with a mortality rate of up to 14.7% [1]. They mostly occur in the gastrojejunal anastomosis or along the gastric vertical staple line [2], with an estimated incidence of 0.7-5% [3]. Despite recent advances in treatment modalities, it remains a therapeutic challenge and often requires clinical intensive care, multiple radiological, endoscopic, and surgical procedures, and interdisciplinary involvement [3, 4].
Endoscopic techniques are now considered the first-line approach for the management of bariatric surgery-related fistulas, as they spare many patients who would otherwise undergo revisional surgery at an increased risk of adverse events [3]. A variety of different procedures and devices has been proposed with variable success rates, including clips, mesh plugs, self-expandable metal stents, tissue sealants, suturing platforms, internal drainage, vac-uum therapy, brushes, and argon plasma coagulation [3, 5-7].
The use of cardiac septal defect occluders (CSDO) is an emerging technique that has demonstrated favorable outcomes for the closure of extravascular defects, such as bronchopleural, tracheoesophageal, enteroatmospheric, and rectovaginal fistulas [5]. Other studies have reported similar results with the CSDO AmplatzerTM for the management of GI disruptions following bariatric surgery [8]. However, the use of alternative devices for this purpose has not yet been described. The current report presents the first off-label use of the Occlutech® occluders for the treatment of a chronic fistula after bariatric revisional surgery.
Case Report
A 52-year-old male with a body mass index of 23.6 kg/m2 and no comorbidities was referred to our institution for surgical consultation due to a chronic post-bariatric gastrocutaneous fistula. His bariatric history started in 2002, after he underwent an open modified Scopinaro procedure at an initial weight of 216 kg (BMI 57.4 kg/m2). Despite midterm satisfactory results (44% total weight loss), the patient presented with weight reganance and an incisional hernia. Bariatric revisional surgery and hernia repair were performed in 2017 at a weight of 170 kg (BMI 45.1). He soon developed a gastric pouch leak with multiple intra-abdominal collections and sepsis, which required a long period of conservative management with open abdomen and negative wound pressure therapy, parenteral nutrition, and intravenous antibiotics.
Over the course of several months, he developed an epithelized gastrocutaneous fistula with controlled but persistent drainage out of the proximal edge of his planned ventral hernia (Fig. 1a). An upper endoscopy identified a fistulous orifice at the proximal edge of the vertical staple line, just below the esophagogastric junction, measuring approximately 8 mm (Fig. 1b), with extraluminal extravasation that led to a recurrent left subphrenic abscess (Fig. 1c).
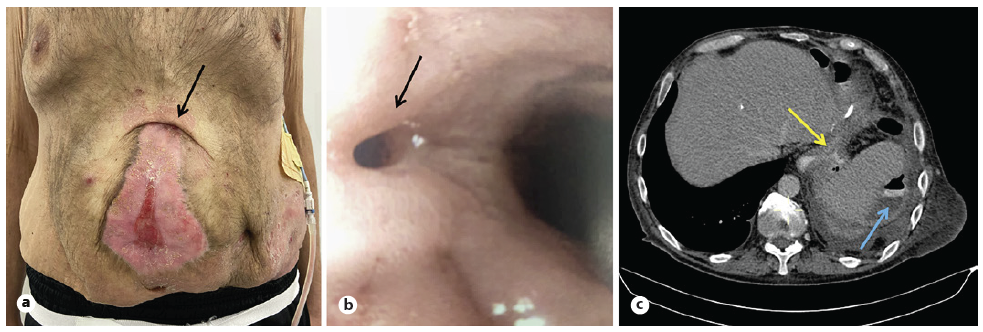
Fig. 1 a Clinical photograph (arrow: cutaneous orifice of fistula; pigtail drain in left subphrenic abscess). b Endoscopic image of the fistula opening (arrow) below gastroesophageal junction. c CT scan: extravasation of oral contrast media (yellow arrow); left subphrenic abscess (blue arrow).
Endoscopic treatment was considered the preferred choice for this case, given the poor nutritional status and hostile abdomen. For years, he had undergone multiple attempts at fistula closure using argon plasma coagulation, internal and external drainages, clipping, fibrin sealants, evac therapy, and stenting.
Discouraged with multiple failed repair attempts, he was referred for further evaluation in our center. After a multidisciplinary team discussion, a decision was made to proceed with an innovative endoscopic technique. The placement of a CSDO across the fistula orifice was planned with the agreement and written consent of the patient.
An Occlutech® muscular VSD occluder (Occlutech International AB, Helsingborg, Sweden) was selected due to the long funnel-shaped aspect of the defect, similar to a ventricular septal defect. This device consists of a braided nitinol disc designed to adapt to the shape of the defect and effectively achieve immediate closure. The patch material also serves as a matrix for subsequent tissue ingrowth and granulation that may contribute to fistula closure [9].
The procedure was performed in the catheterization laboratory under intravenous sedation and topic anesthesia. The fistula was cannulated from the esophagus by a biliary stent deployment system under direct endoscopic guidance and the extraluminal leakage was documented by contrast injection. An AmplatzTM extra stiff guidewire (Cook Medical, Bloomington, IN, USA) was inserted through the fistula orifice and its adequate position was con-firmed by fluoroscopy. The delivery system was introduced over the guidewire and the CSDO was deployed under endoscopic and fluoroscopic guidance with no immediate adverse events (Fig. 2a). A contrast study after the CSDO placement demonstrated no extravasation of contrast material through the device.
After the procedure, restricted oral intake was required for 24 h. The patient was placed on a liquid diet for 10 days and was advanced to a regular diet on day 12, despite the 10-15cc remaining daily drainage through the pigtail drain positioned at his left subphrenic abscess. Nearly 6 weeks later, the pigtail was accidentally displaced, and the patient progressively developed systemic signs of sepsis. Computed tomography and fluoroscopy documented recurrence of the abscess and partial dislodgment of the 8-mm mVSD CSDO (Fig. 2b). The device probably tore the friable tissue around the fistulous orifice and got stuck in the tunnel-shaped tract, and it was not possible to see or snare the device by endoscopic view. Given the apparent (though limited) success of the device, a second attempt with an oversized disc (Occlutech® Figulla Flex II UNI 24-mm) was made (Fig. 2c), ultimately sealing the fistulous orifice with the former device positioned between the two discs of the new one (Fig. 3a).
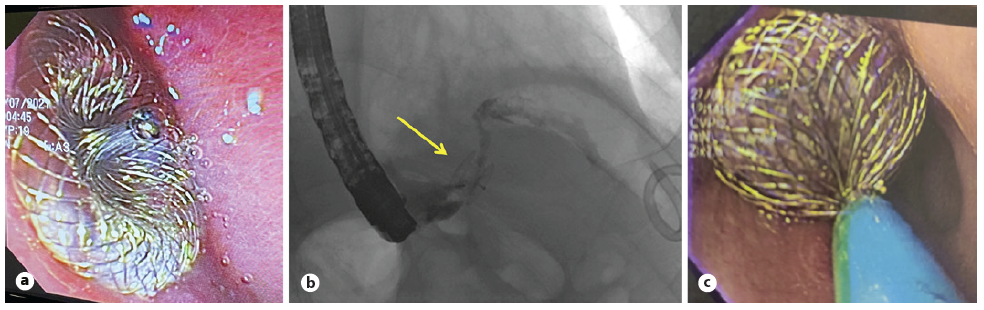
Fig. 2 a Endoscopic visualization of the first device in place - Occlutech® mVSD occluder. b Fluoroscopic image after partial dislodgement of the device; fistula cannulation demonstrating extraluminal extravasation of contrast material through the occluder. c Endoscopic visualization of the second device in place - Occlutech® Figulla Flex II occluder.
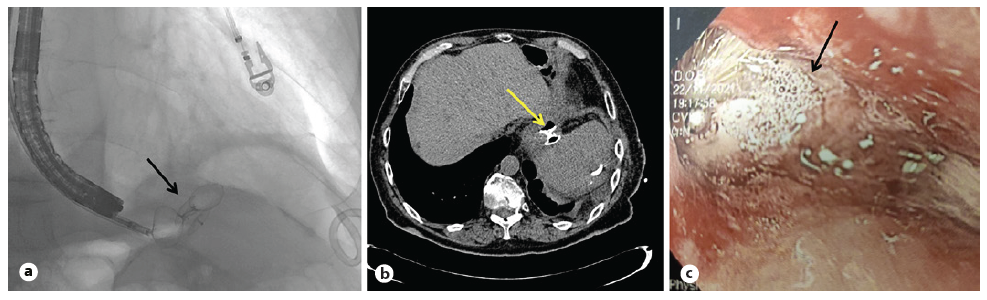
Fig. 3 a Fluoroscopic image showing the first device positioned between the two (luminal and extraluminal) discs of the second one. b CT scan with both devices in place, with no extravasation of oral-administered contrast media. c Endoscopic visualization of both devices engrafted.
At the 6-month clinical and imaging follow-up, upper endoscopy and contrast-enhanced CT scan showed the device already engrafted and a significant reduction of the chronic abscess, with no signs of fistula recurrence (Fig. 3b, c). The pigtail was maintained in the subphrenic space to monitor any sign of fistula recurrence and occasionally drained debris from the chronic abscess. It was finally removed after the follow-up imaging, and no drainage was observed from its insertion orifice or the previous cutaneous fistulous tract.
Discussion/Conclusion
The concept of the use of CSDO for the treatment of GI fistulas is not novel, albeit formally it is considered an off-label indication. Its use in the setting of post-bariatric fistula treatment is still very limited to a small number of case reports [5, 10-12]. To the best of the authors’ knowledge, no reports on the use of the Occlutech® devices for this purpose are available comparable to former studies with the AmplatzerTM Occluders.
Early results suggest this technique is particularly useful for poor surgical candidates with chronic GI fistulas that have had failure of closure attempted with standard endoscopic methods [8]. In these cases, the persistent inflammation, fibrosis, and epithelialization of the tract is often not liable to the application of clips, sutures, sealants, and conventional ablative techniques, leading to failed closure attempts in up to 20% of cases [12].
In the presented case, multiple attempts using endoscopic techniques have ultimately failed due to a mature fistula tract and a chronic adjacent abscess. Nevertheless, a new application method of the Occlutech® endoscopic device has obviated the clinical burden of a high-risk laparotomy, providing a more suitable alternative to surgical repair.
This case also enlightens the importance of perseverating on minimally invasive modalities to manage these challenging cases. It is also of note that appropriate endoscopic equipment, as well as the involvement of a multi-disciplinary team comprising of advanced endoscopists, surgeons, interventional radiologists, and interventional cardiologists, are prime conditions to ensure successful patient outcomes.
In conclusion, this report has successfully demonstrat-ed the technical feasibility, safety, and efficacy of the Occlutech® occluders for the endoscopic treatment of a chronic gastrocutaneous fistula. Given that the long-term efficacy of the off-label use of CSDO for GI fistula closure is unknown, further trials are expected to assess and compare different devices and other treatment modalities.














