Introduction
Eosinophilic gastrointestinal disorders (EGID) are rare conditions characterized by infiltration of the gastrointestinal tract (GIT) by eosinophils, in the absence of known causes for eosinophilia (e.g., parasitic infection, malignancy, drug reactions, …) [1].
Although idiopathic in nature, an allergic mechanism has been suggested in at least a subset of these patients. In fact, a personal or family history of food allergies, asthma, and atopic disorders is present in about 50% to 70% of cases [2]. The pathogenesis of the disease is not well defined, but it is thought to be a polygenic allergic disorder on the spectrum between IgE-mediated and delayed Th2 responses, in which an alteration of the mucosal integrity allows the direct contact of antigens with the gut wall, triggering an eosinophilic inflammatory response [3].
This eosinophilic infiltration can occur in any area of the GIT - the antrum and proximal small bowel being the most frequently affected segments - and throughout the different layers of the GIT: mucosal (the most frequent), muscular, or serosal, so that the area and depth of the eosinophilia determine the clinical presentation [4-6]. Thus, patients with mucosal disease may present with abdominal pain, diarrhea, bleeding, iron-deficiency anemia, malabsorption symptoms, proteinlosing enteropathy, or failure to thrive; the muscularis involvement can cause bowel thickening, leading to obstruction, and predominantly serosal pattern, which occurs in a minority of patients, presents with exudative ascites [1, 7].
EGID may or may not be accompanied by peripheral eosinophilia or elevated serum IgE levels and has unspecific radiologic findings, so that, together with clinical, laboratory, and endoscopic findings, the histologic confirmation of eosinophilic infiltration is necessary for a definite diagnosis [1, 8].
We describe the case of a patient with eosinophilic ileitis (EI) with transmural inflammation.
Case Presentation
A 40-year-old male presented in the emergency department, complaining of generalized abdominal pain, almost every day for 1 month, with no relation with meals. He also presented with nausea and vomiting, diarrhea (3-4 liquid stools), anorexia, and an unintended 10-kg weight loss in 2 months. He denied fever, dysphagia, fecal mucus, or bleeding. He was previously medicated with paracetamol, ibuprofen, scopolamine, and omeprazole, without a favorable response.
He worked as a brickworker, was an active smoker, and his only antecedents were dyslipidemia, removal of a sacrococcygeal cyst and an anal fistulotomy. He denied chronic medication, consump-tion of non-piped water, or contact with non-dewormed animals. He had no personal or family history of allergic or gastrointestinal pathologies.
His only significant finding on physical examination was mucosal pallor. Analytically, he had acute anemia (hemoglobin concentration of 12.5 g/dL, with a normal level from 2 days earlier), peripheral eosinophilia (1,600 eosinophils/L), and slight hypokalemia (3.4 mEq/L). The remaining results were normal, including He performed an abdominal ultrasound that showed a “small amount of free intraperitoneal liquid” and “several bowel loops demonstrating parietal thickening” (shown in Fig. 1) that was clarified with a thoracoabdominal CT scan that revealed a “thin sheet of bilateral pleural effusion, reduced-volume ascites and extensive regular parietal thickening of the entire ileum.”
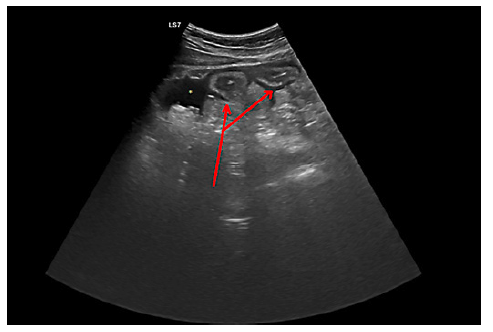
Fig. 1. Radial EUS demonstrating two small bowel loops with a thickened wall (red solid arrow), surrounded by free peritoneal fluid (asterisk).
He was then hospitalized for symptomatic therapy, correction of hypokalemia, and diagnostic investigation. Analytically he had an elevated fecal calprotectin (974 μg/g); elevated serum IgE was absent and stool for parasites or bacteria were negative, such as viral serologies, mycobacteria direct exam and culture, and celiac disease screening.
He performed an upper endoscopy and colonoscopy, both of which showed no macroscopic lesions, including 5 cm of visualized ileum. Histopathological analysis revealed gastritis with Helicobacter pylori and “lymphoplasmocytic infiltrate, including eosinophils (fewer than 10 per high-power field HPF]), with signs of mild activity” in the ileal specimens. Then a CT enterography revealed similar results, namely “moderate edema of the ileum walls and small volume ascites, with no appreciable changes in the colon,” as shown in Figure 2.

Fig. 2. CT enterography revealing a thickening of different layers of the ileal wall. The asterisk indicates a small amount of ascites.
After 13 days of supportive treatment, the patient maintained 3-6 daily stools and peripheral eosinophilia (1,300 eosinophils/L), even though there was a resolution of the anemia and hypokalemia.
An antegrade motorized spiral enteroscopy (MSE) was performed, where the only macroscopic alteration was a single erosion in the distal jejunum (shown in Fig. 3). Despite the normal appearing mucosa, shown in Figure 3, ileal random biopsies were collected and histological analysis revealed a “very fragmented small bowel mucosa, with inflammatory infiltrate with about 35 eosinophils per HPF (and about 10 eosinophils per HPF in crypt epithelium), without epithelial permeation, microabscesses or erosion, being compatible with eosinophilic enteritis” (shown in Fig. 4).
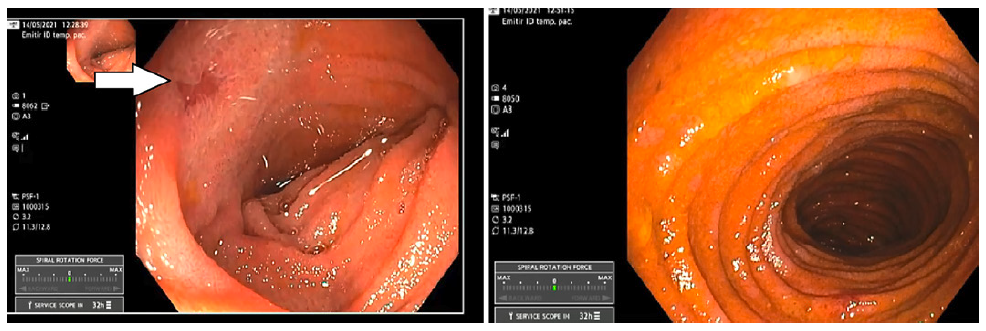
Fig. 3. A single small erosion detected in the distal jejunum (left) and the normal appearing ileal mucosa, observed by the antegrade motorized spiral enteroscopy.
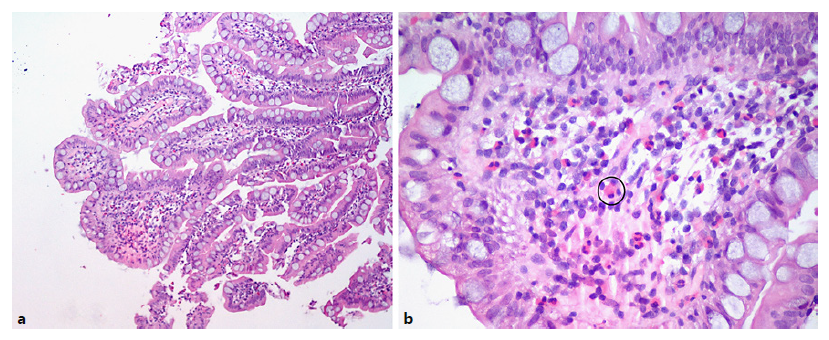
Fig. 4. a Ileal wall showing a fragmented mucosa, with inflammatory infiltrate. Hematoxylineosin. Original magnification, ×10. b Amplification of intestinal villi, showing the presence of eosinophils (one example surrounded by a black circle). Hematoxylineosin. Original magnification, ×40.
The patient initiated on treatment with oral prednisolone and was discharged after 17 days, with clinical improvement (absence of abdominal pain and 1-2 daily stools) and a normal analytic study. He completed a course of 4 weeks of corticotherapy, which has proven to be successful at 1-month clinical and imagiological re-evaluation.
Discussion/Conclusion
The most common cause of ileitis is Crohn’s disease; however, it can be caused by a wide variety of other conditions, such as infectious diseases, vasculitis, neoplasms, drug-induced and others, including eosinophilic enteritis [1]. The latter belongs to EGID and should be considered in the differential diagnosis of unexplained GI symptoms.
Among EGID, EI is a particularly rare entity, so there have been few epidemiological studies, most of them addressing the prevalence of EI along with eosinophilic gastritis. Thus, in a retrospective study that included over 35 million individuals between 2012 and 2017, the prevalence of eosinophilic gastroenteritis (EoGE) was 5.1/100,000 people, which was similar to the results of Reed et al., in which the overall prevalence of EoGE along with eosinophilic colitis was 2-28/100,000 people (including adults and children), and did not differ much from those of a large retrospective analysis of a database with over 11 million individuals, in which 8.2/100,000 people met criteria for EoGE [9-11]. Even though epidemiologic data are limited, there has been an increasing prevalence and incidence of EGID for unknown reasons, being hypothesized that this trend may reflect the development of new and better diagnostic methods - increasing the detection rate -, along with the growth of allergic pathologies in developed countries, as explained by the hygiene hypothesis [11].
Our patient had particular features, as most of studies verify that EoGE is more prevalent in females and that prevalence gradually decreases with age, being higher in patients younger than 5 years [10]. In addition, several case series and single center studies have identified allergic comorbidities in patients with EoGE, ranging from 30 to 45.6% in adults and going up to 80% in children [12, 13]. As referred, a personal/family history of atopy was absent in this patient, which has concealed the suspicion of an eosinophilic pathology.
Clinically, the symptoms presented by our patient were consistent with those described in literature. According to Jensen et al., when the upper GIT is involved, abdominal pain, diarrhea, nausea, and vomit-ing are the most frequent symptoms, in descending order [10]. In this case, there was also ascites, which implies an involvement of the serosa, being therefore compatible with a transmural eosinophilic infiltration. Differently of eosinophilic pleural effusion - defined as containing at least 10% eosinophils in the white cell differential count -, there are no defined criteria for eosinophilic ascites [14]. Even so, an elevated eosinophilic count in ascitic fluid would have been a valuable diagnostic indicator; however, because of the small volume ascites, performing a paracentesis was technically impossible.
Besides, our patient had ileal wall thickness, which is in agreement with other cases described in the literature, such as Zhang et al. and Zheng et al. [5, 15]. Similarly, endoscopy did not contribute to clarify the diagnosis, such as in Reed et al.’s study, in which 47% of endoscopic examinations were normal, so that it was essential to study the ileal region [16]. With this in mind, methods such as endoscopic videocapsule would be useless and time-consuming since not only the probability of finding results was low, but also it would not allow performing biopsies. Therefore, to allow direct endoscopic access to the ileus, we performed an MSE. The alternative - balloon-assisted enteroscopy (single or double) - is currently the method of choice in most centers, since its efficacy and safety has been extensively studied. The MSE presents a different principle, where the bowel is pulled onto the scope rather than traversed by pushing the scope forward. In a recent systematic review with meta-analyses, the two methods were compared and it was found that despite the fact that there were no significant differences in diagnostic and therapeutic success rates, the procedure time was significantly shorter for the MSE group, with comparable safety [17, 18]. Until this day, we have performed in our center 10 of these procedures, with a high level of efficacy and safety.
To our knowledge this is the first reported case in which the use of MSE allowed the diagnostic of EI, enabling the observation of the entire small bowel and the collection of multiple biopsies from the ileum in about 70 minutes, without complications.
Regarding histopathology, since eosinophils can be detected in normal ileal mucosa, the concept of “abnormally increased eosinophils” is not well defined. According to Collins criteria, up to 28 eosinophils per HPF can be found in lamina propria of normal ileum, with a minimum of 56 eosinophils per HPF necessary for an EI diagnosis. Nonetheless, more recent evidence suggests that counting mucosal eosinophils is of little practical value when compared to the analysis of their distribution; eosinophils are normally solely dispersed in the lamina propria so that even a few cells in other locations should raise EI suspicion. Therefore, more than the 35 eosinophils per HPF, we valued the presence of epithelial cell damage along with the altered eosinophil distribution - with more than 4 eosinophils per HPF in crypt epithelium - and the absence of acute inflammatory cells [19, 20].
Although there are no consensus recommendations regarding the diagnosis, we supported it on orientations described in the literature, namely the conjugation of clinical manifestations with peripheral eosinophilia, an altered eosinophil distribution in ileal wall, the lack of involvement of other organs, and ultimately the favorable response to corticotherapy. Since the patient presented with peripheral eosinophilia (>1,500/L on admission) and organ disfunction, we consider of uttermost importance his surveillance with regard to the development of extragastrointestinal symptoms that may suggest that this is an initial manifestation of a hypereosinophilic syndrome, which implies a distinctive therapeutic approach and prognosis [21, 22].
In conclusion, EI is a rare condition that should be considered in the differential diagnosis of unexplained GI symptoms and ascites, particularly in the presence of peripheral eosinophilia. We report an unusual case of EI with transmural eosinophilic inflammation, in which MSE had an essential role for a less time-consuming diagnosis.















