Introduction
Eosinophilic gastroenteritis (EoG) results from excessive infiltration by eosinophils in the mucosa, submucosa, or muscularis of the stomach and small bowel [1, 2]. It is a rare condition with an estimated prevalence of 5.1 per 100,000 people in the USA [3]. Pathophysiology is poorly understood, but atopy is a known risk factor [1, 3, 4]. Here, we report a case of a young patient with a history of atopy presenting with ascites, constitutional symptoms, and peripheral eosinophilia with a delayed diagnosis of EoG.
Case Report
A 36-year-old woman with a medical history of asthma and urticaria under treatment with levocetirizine and a combined oral contraceptive was admitted to the hospital for the study of acute diarrhea (1-2 liquid stools per day with no blood or mucus), associated with abdominal discomfort, abdominal swelling, early satiety, and asthenia with onset 2 weeks before. The patient also reported wheezing episodes on the previous week. During this period, she looked for medical assistance three times being discharged with symptomatic treatment and cotrimoxazole with no improvement. Physical examination revealed normal blood pressure (122/64 mm Hg) and heart rate (77 bpm), no fever (auricular temperature 36.5°C), 98% peripheral oxygen saturation, no mucocutaneous alterations, and cardiac and pulmonary auscultation without alterations, with moderate volume ascites as the only remarkable finding.
Laboratory results (Table 1) showed peripheral eosinophilia (7,000 eosinophils/μL, 45% of the leucocytes, ref. range <500/μL) and increased seric immunoglobulin E (1,083 IU/mL, ref. range <129 IU/mL). An abdominal ultrasound revealed diffuse small bowel wall thickening and moderate volume ascites. The cell count of the ascitic fluid revealed 89% of eosinophils (8,337/μL, Table 2). Serum ascites albumin gradient was inferior to 1.1, suggesting a peritoneal cause for the ascites. A thoraco-abdominopelvic computerized tomography presented no lung findings (Fig. 1a), yet it was identified a slight thickening of the distal esophagus (Fig. 1b), and diffuse jejunal and ileal wall thickening (Fig. 1c), without lymph node enlargement. An upper and lower endoscopy with segmental biopsies was performed, revealing a normal endoscopic appearance of the mucosa (Fig. 2). At this point, the considered differential diagnosis was EoG, eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), hypereosinophilic syndrome, and, less likely, parasitosis, infectious gastroenteritis, food or drug hypersensitivity, celiac disease, connective tissue diseases, inflammatory bowel disease, and hematologic neoplasia [1, 2].
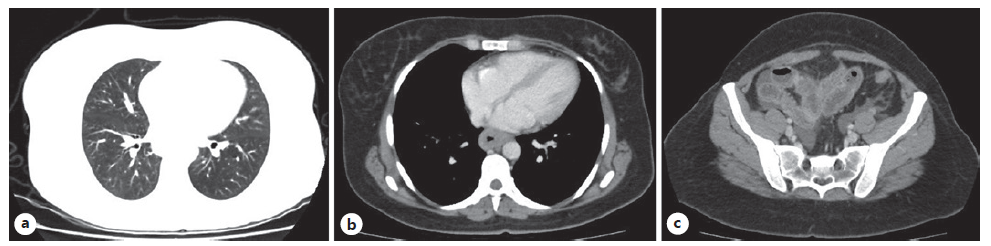
Fig. 1. Thoracoabdominopelvic computerized tomography (CT) without lung alterations (a); distal esophagus wall thickening (b); and diffuse jejunal and ileal wall thickening (c).
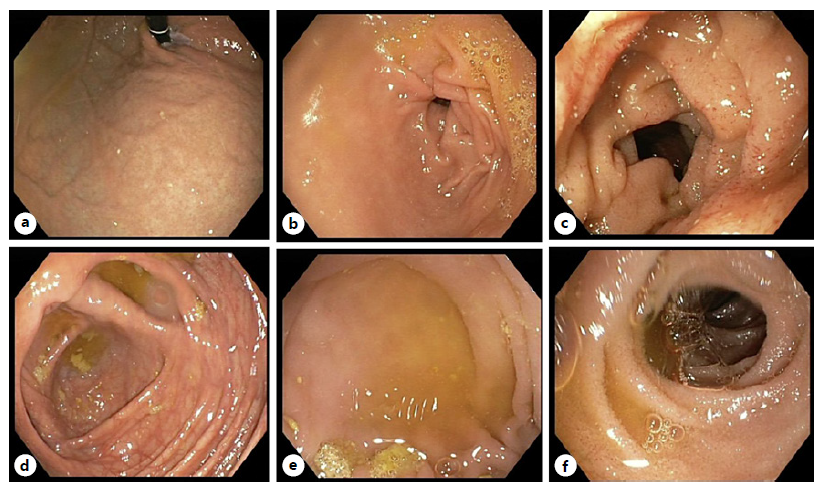
Fig. 2. Upper GI endoscopy with normal endoscopic appearance of gastric cardia and fundus (a), gastric antrum (b), and duodenum (c). Colonoscopy with ileoscopy with normal mucosa of the cecum (d, e) and terminal ileus (f).
An exhaustive screening for systemic eosinophilia causes was carried out. The peripheral blood smear did not reveal cellular atypia. Serum levels of vitamin B12 were normal - 441 pg/mL (ref. range 211-911 pg/mL), such as folate 7.2 ng/mL (ref. range 3.5-17.5 ng/mL); serum iron was slightly below normal - 31 μg/dL (ref. range 50-170 μg/dL). Serum ferritine, total ironbinding capacity (TIBC), and transferrin saturation presented normal values. Microbiologic and parasitological stool examinations were negative for pathogenic strains. Serum antibodies anti-toxocara canis, anti-echinococcus, and anti-fasciola were also negative. The immunological study revealed negative antinuclear antibodies and antineutrophil cytoplasm antibodies. There was a slight elevation of the inflammatory marker C-reactive protein (12.9 mg/L; ref. range <3) with normal erythrocyte sedimentation rate. Anti-VIH I/II anti-bodies were negative. Total serum immunoglobulin E was increased (1,083 UI/mL; ref. <129). Immunoglobulin subtypes other than IgE and serum protein electrophoresis were within the normal range. The inhalatory allergen panel (Rast IgE) was positive, yet the food allergen panel was negative. Skin prick tests were negative for food allergens and positive for dust mites, dog and grass allergens. Electrocardiogram presented a sinusal rhythm without alterations. Troponin I and CK-MB seric levels were normal. Spirometry presented a mild restrictive ventilatory pattern. Liver tests were normal except for slightly elevated ALT levels at admission, which normalized 3 days later. Renal dysfunction markers and urinary sediment were within the normal range. In sum, an extensive investigation of the alternative causes of peripheral eosinophilia and infiltration of other organs by eosinophils was negative.
Empirical treatment for EoG with oral prednisolone 40 mg per day and the six-food elimination diet (eggs, milk, soy, nuts, seafood, wheat) was initiated with the support of a dedicated nutritionist. A rapid clinical improvement was observed with normalization of bowel habits and a reduction of abdominal volume. Additionally, a rapid reduction in peripheral eosinophilia with normalization at 3 weeks was observed.
Posteriorly, results of the endoscopic biopsies confirmed eosinophilic infiltration of the duodenum and ileum (>50 eosinophils per high-power field [HPF]), less expressive on esophageal and colonic biopsies (10 eosinophils/HPF; Fig. 3), which sustains the diagnosis of EoG. Gastric biopsies were negative for Helicobacter pylori.
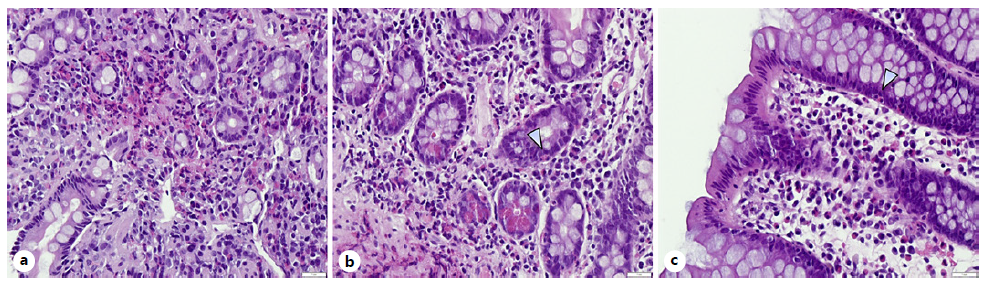
Fig. 3. Histopathologic images of endoscopic biopsies of duodenum (a) and ileum (b), HE. ×400, with infiltration of the lamina propria with eosinophils (>52 per HPF). Esophageal and colonic (c) biopsies with >10 eosinophils/HPF. HE, hematoxylin and eosin coloration.
Three months after the initial episode and upon the terminus of the oral steroid course, the patient was in clinical and laboratorial remission and so a progressive reintroduction of eliminated foods was conducted with close monitoring. It was observed a resurgence of diarrhea and abdominal pain with seafood ingestion ceased with subsequent restriction of these foods in the patient’s diet. Two years after the diagnosis, the patient repeated upper endoscopy and colonoscopy with ileal and colonic biopsies, which revealed no eosinophilic infiltration. Three years after the initial episode, the patient is asymptomatic on a diet with a restriction of seafood without relapses or the need for any treatments.
Discussion
EoG is a rare condition with a yet poorly understood pathophysiology [1-3]. Clinical presentation depends on the site, extent, and depth of disease in the gastrointestinal (GI) tract [5]. Patients often present with nonspecific GI symptoms that may be accompanied with peripheral eosinophilia [1]. When there is serosal infiltration, patients often present with ascites associated with nonspecific GI symptoms such as abdominal pain, nausea, or vomiting [1]. Diagnosis is made by infiltration of the GI tract by excessive numbers of eosinophils in the absence of alternative causes [6]. The number of eosinophils necessary for diagnosis is not well defined, yet recent reviews suggest the values of >30 eosinophils per HPF for eosinophilic gastritis, >52 eosinophils per HPF for enteritis, and for eosinophilic colitis, >50 per HPF in the right colon, >35 per HPF in the transverse colon, and >25 per HPF in the left colon [1, 7]. The most relevant differential diagnosis is hypereosinophilic syndrome with GI involvement and secondary eosinophilic infiltration, which may occur in GI infections, hypersensitivity reactions, or connective tissue diseases [1, 2].
First-line treatments are short courses of systemic corticosteroids and food elimination diets with good efficacy [8]. Prognosis is generally favorable for patients who respond to first-line treatments, yet a minority of patients can exhibit a relapsing and remitting course and, therefore, EoG patients should receive long-term monitoring [8, 9]. The optimal follow-up is still a matter of debate with limited supporting data [1, 8]. The authors propose a close monitoring (every 4 weeks) until remission is achieved, followed by a further spaced follow-up (e.g., every 6 months). Remission can be assessed by the absence of symptoms and peripheral eosinophilia that can be complemented with repeated endoscopic biopsies when there are doubts. Despite being diagnosed by exclusion, it is important to suspect EoG with subserosa involvement in patients presenting with the uncommon association of peripheral eosinophilia and ascites, particularly if there is a history of allergies.