Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the seventh leading cause of cancer death world-wide with 495,773 new cases and 466,003 deaths reported in 2020 [1]. In the next few years, PDAC is estimated to become the second cause of cancer mortality in developed countries - including in Portugal where annual deaths should surpass 2,000 (n = 2,137; 95% CI, 1,862-2,413) by 2035 reflecting an increase of 51% [2-4]. Without treatment, median survival of metastatic PDAC (mPDAC) lies between 3 and 6 months. Although few patients (15-20%) are amenable to surgery combined with adjuvant chemotherapy, overall survival is of 11-25 months [5, 6].
The mainstay of current therapeutic approach for mPDAC is the combination of cytotoxic drugs such as FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, oxaliplatin), gemcitabine with nab-paclitaxel, and gemcitabine plus capecitabine [7-10]. Additional attempts to further improve survival include modifying the sequence of combination regimens, adding other cytotoxic agents, or performing maintenance strategies [11-13].
The recent implementation of target therapies was especially important for the management of pancreatic tumors, given that an unstable genotype with numerous structural variations (e.g., BRCA1, BRCA2, or PALB2 genes or mutational signatures of DNA-damage repair deficiency) is present in about 10-15% of patients. Germline mutations in BRCA genes are identifiable in around 4-7% of patients with mPDAC [14-16]. As these cells with a deficient DNA repair are usually sensitive to adenosine diphosphate-ribose polymerase (PARP) inhibition [17, 18], there is growing role for PARP inhibitors as potential therapies [19-21]. The efficacy of the PARP inhibitor olaparib as a single agent in germline BRCA mutation and PDAC was initially suggested in a phase II trial that demonstrated median progression-free survival and overall survival rates of 4.6 and 9.8 months, respectively [19]. The effect of olaparib as a maintenance therapy in patients who had a germline BRCA1 or BRCA2 mutation with mPDAC not progressing during the first-line platinum-based chemotherapy was assessed in the phase III POLO trial [22] showing progression-free survivals of 7.4 months versus 3.8 months in placebo (p = 0.004) [23]. Based on these findings, olaparib was approved by the US Food and Drug Administration (FDA) on December 2019 and by the European Medicines Agency (EMA) in July 2020 and is currently recommended by the ASCO guidelines (2020) for the maintenance treatment of adult patients with germline BRCA mutations mPDAC whose disease has not progressed during at least 16 weeks of the first-line platinum-based chemotherapy [24]. Other drugs that are being currently tested in this scenario include nimotuzumab (anti-EGFR), durvalumab (anti-PDL-1), nivolumab (anti-PD-1) [13].
Nonetheless, the late integration of target therapies in clinical practice accentuates the need for multidisciplinary and consensual decision-making processes, which arouses new challenges, as well as changes in work routines, that are daily faced by pancreatic cancer specialists. Several questions regarding patients’ journey still need to be clarified, including the use of new techniques for disease early diagnosis and staging (e.g., endoscopic ultrasound-guided fine-needle biopsy - EUS-FNB), identification of biomarkers and criteria that influence therapies’ selection [25-28]. Thus, the aim of this study was to characterize the initial healthcare journey of mPDAC patients in Portugal (after reaching a referral center), including healthcare provision, and major factors currently affecting therapeutic decisions, namely BRCA mutations testing.
Materials and Methods
Study Design and Variables
A descriptive cross-sectional web-based survey (Google form) using a convenience sampling approach was performed. Portuguese oncologists and pathologists that routinely work with mPDAC patients from the different geographical regions and settings (public, private hospitals) were invited to participate in the study via email (December 2020) (the list of potentially eligible physicians with clinical practice experience in this field was provided by the Grupo de Estudos em Cancro Digestivo-GECD - Portugal). Physicians were fully informed regarding the nature of the study, the procedures for data recording, and the voluntary nature of their participation. Responders provided their informed consent before survey’s completion, and anonymity was guaranteed. Participants’ withdrawal was allowed at any time. This study was waived of bioethical approval (National Legislation-Law 21/2014) because it does not contain any intervention on human subjects nor individual health data collection.
The questionnaire was divided into two sections that aim to assess physicians’ perception on mPDAC patients journey in Portugal in the previous year, 2019 (i.e., to avoid bias from COVID-19 potential disruptions in health services). The first section (18-items) was answered by both oncologists and pathologists and included information on: sociodemographic data (e.g., medical specialty, working place), current histopathological diagnosis, disease staging procedures, and biomarkers evaluation. The second part of the questionnaire (12-items) was intended only for oncologists and covered topics on therapeutic approaches and complementary procedures for mPDAC management.
The questionnaire was specifically developed for this study by the Coordinator Committee (Anabela G. Barros [oncologist], Hélder Mansinho [oncologist], Nuno Couto [oncologist], Manuel R. Teixeira [pathologist], Filipa Duarte-Ramos [pharmacist, epidemiologist]). Responders took an average time of 10 min to complete the survey. Standards for scientific research were performed according to the Declaration of Helsinki. The complete questionnaire (original language, Portuguese) is available in online supplementary Appendix (for all online suppl. material, see https://doi.org/10.1159/000533178).
Data Analysis
The normality of the variables was assessed using Kolmogorov-Smirnov and Shapiro-Wilk tests with additional visual inspection of Q-Q plots. Descriptive statistics were used to summarize the data, with absolute and relative frequencies to describe categorical variables and median and interquartile range (IQR) for continuous non-normal variables. The association between categorical variables was assessed through Pearson’s χ2 test (alternatively, when few observations, e.g., less than 5, exist, the Fisher’s exact test was used). Analyses were conducted in Stata Statistical Software version 15.0 SE (College Station, TX: StataCorp LL, USA) and p values below 0.05 were considered statistically significant.
Results
Diagnosis of mPDAC: Oncologists and Pathologists’ Overview
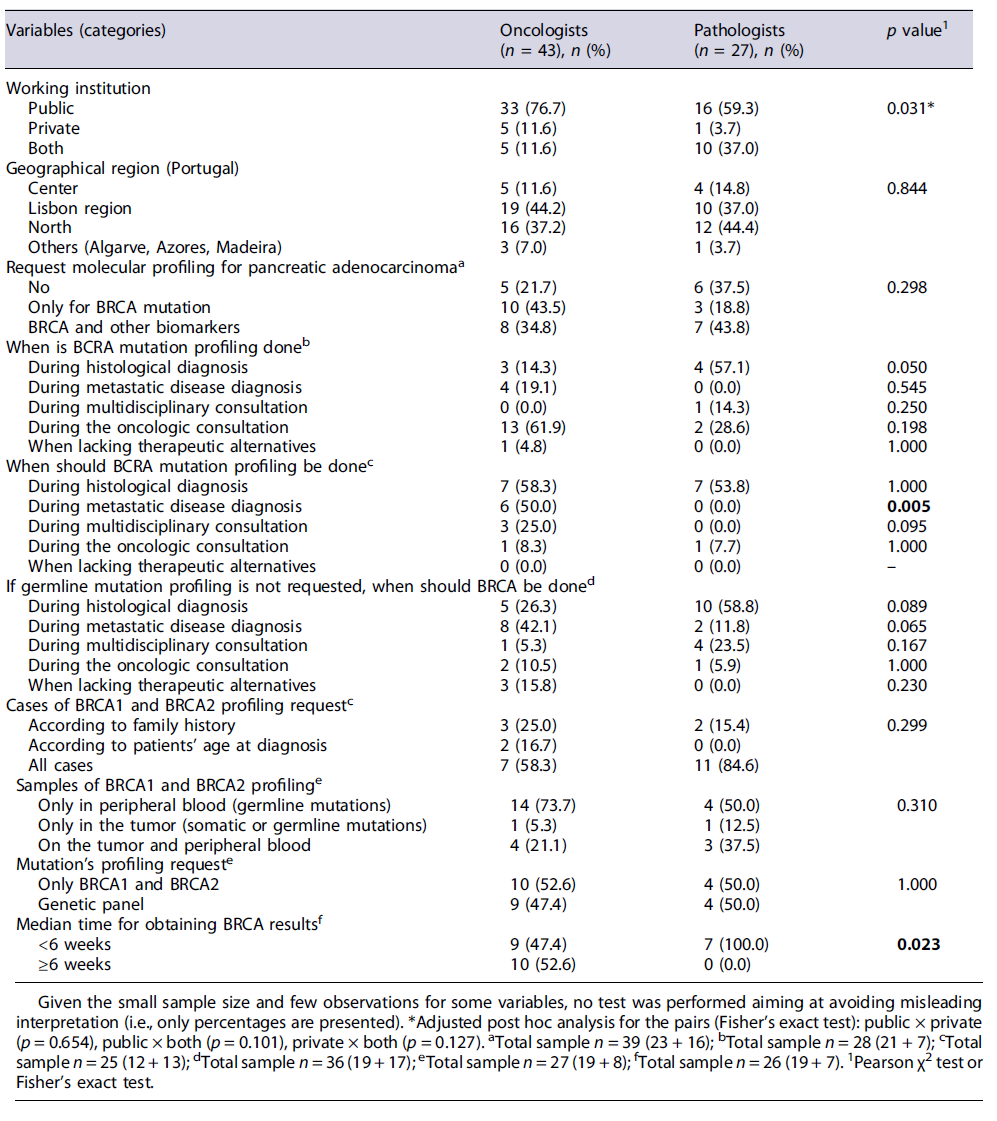
Overall, 70 physicians (from n = 34 invited Centers in Portugal) participated in the study, of which 43 were oncologists and 27 were athologists, mostly from Lisbon and Vale do Tejo (41.4%) and North (40.0%) regions. Table 1 shows the sociodemographic characteristics of the participants and their perception on the initial journey of patients with mPDAC in Portugal. Most physicians (n = 49; 70.0%) work only in public health institutions. Although oncologists may have a greater representation in this setting (76.8% vs. 59.3% of pathologists), pathologists also labor in both public and private centers (37.0% vs. 11.6% of oncologists). The most frequent types of pancreatic cancer diagnosed in the physicians’ institutions are adenocarcinoma-found in 90% of patients, followed by undifferentiated tumors (5%). Adenosquamous carcinoma and cystadenocarcinoma are poorly reported (around 2% of cases). According to the clinicians, in the past year (12 months - perception over the period from Jan to Dec 2019), a median of 28 patients (IQR 12-70) was diagnosed with PDAC in their center; 22 (IQR 8-70) of them referred to mPDAC. Endoscopic procedures, like ERCP (endoscopic retrograde cholangiopancreatography) or endoscopic ultrasound, complemented with cytology or forceps biopsy sampling, are performed in a median of 50% of admitted patients (IQR 30-70) as part of the histological diagnosis. Metastasis biopsy (percutaneous) is performed in around 50% of cases (IQR 30-70). Almost half of the oncologists (43.5%) request only BRCA mutation as molecular/genetic profiling for mPDAC, while pathologists (43.8%) additionally request the assessment of other biomarkers (e.g., PALB2, ATM, MLH1, MSH2, MSH6 e CDKN2A). Yet around one-third of pathologists stated they do never request this procedure (37.5%) (see Table 1). According to most physicians (n = 18/27; 66.7%), BRCA1 and BRCA2 profiling are done in all cases regardless of patient’s age or family history, using only peripheral blood samples for germline mutations.
According to most oncologists (61.9%), BRCA mutation profiling request is usually performed during the oncologic consultation in their centers, which is slightly different from the perceived clinical routine reported by the pathologists (see Table 1). Most physicians (n = 14 out of 25 responding to this question; 56.0%) recommended BRCA testing to be requested earlier - upon histological diagnosis, especially because the overall median time for results is of 4.0 weeks (IQR 4-8). Yet half of the oncologists (52.6%) believe that this median time is usually over 6 weeks, while for all the responding pathologists this procedure is significantly faster, occurring within 6 weeks (p = 0.023). It was estimated that around 5% of all patients diagnosed in the physicians’ centers present germline mutations. Most pathologists agree that BRCA test should be done during histological diagnosis (58.8%) or requested upon multidisciplinary consultation (23.5%) if germ-line mutation profiling is not requested at that time; conversely, around 40% of oncologists believe this procedure should be performed at diagnosis of meta-static disease (see Table 1).
Management of mPDAC
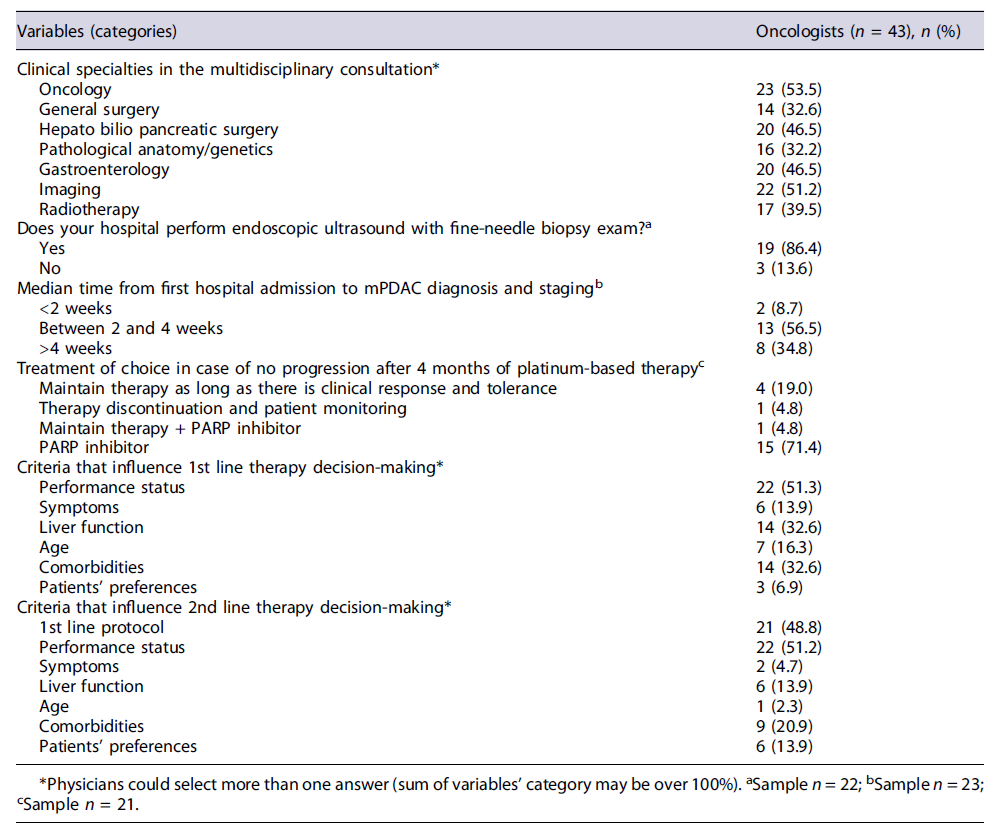
Oncologists’ Perception Oncologists (n = 43) additionally described the current practices for mPDAC management in Portugal, which are depicted in Tables 2, 3. Most physicians state that Oncology (53.5%), Imagiology (51.2%), Gastroenterology (46.5%), and Hepato bilio pancreatic surgery (46.5%) are part of multidisciplinary teams, being other specialties less frequently reported.
Over 65% of oncologists are satisfied or very satisfied with the support provided by different hospital services for the management of the oncologic patients, especially with nursing staff and pain units. Yet around one-third of experts believe that there is room for improvement in the nutrition and palliative care units (see Fig. 1).
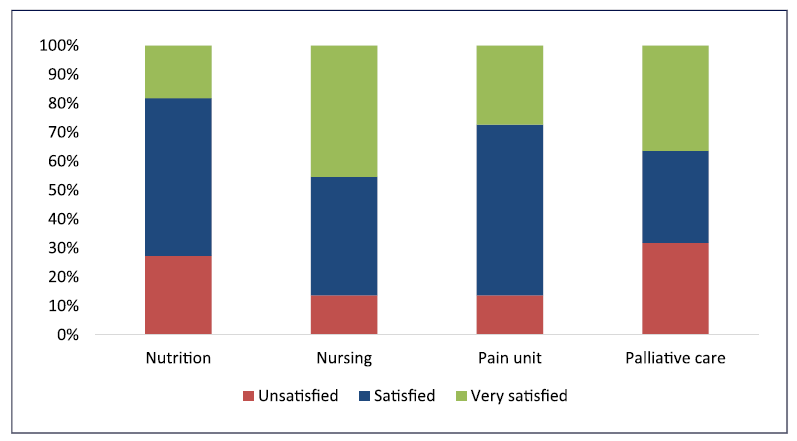
Fig. 1 Oncologists’ perception on the support provided by the different services on mPDAC patients’ management.
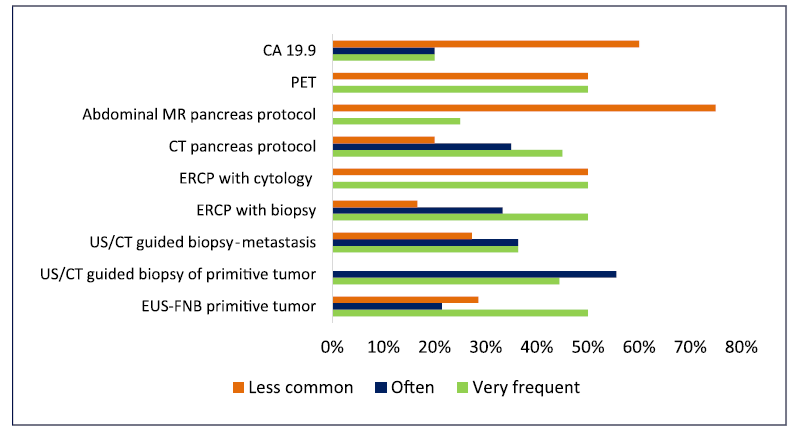
EUS-FNB is available in most institutions/hospitals (according to 86.4% of clinicians) as primary diagnosis approach, being the results generally released in less than 2 weeks-even if performed outside the physicians’ hospital (Table 2). Yet, this procedure (EUS-FNB) for primitive tumor and others such as PET-CT and CT pancreas protocol are often required as complementary exams (according to around 40-50% of physicians) for disease diagnosis and staging. ERCP was also frequently mentioned by the physicians as a complementary procedure, although this is not mandatory for pancreatic cancer. Conversely, abdominal MR and CA 19.9 are rarely performed as complementary procedures (see Fig. 2).
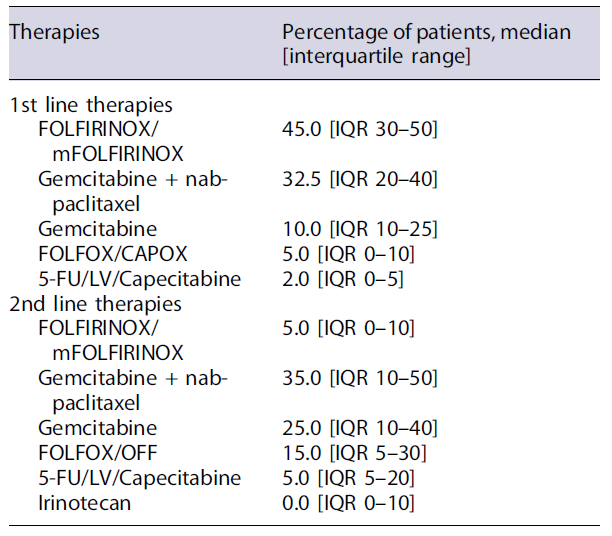
According to the experts, median time from first hospital admission until mPDAC diagnosis and staging is usually between 2 and 4 weeks. PARP inhibitors, when approved, would be the therapy of choice for most oncologists (71.4%) for patients with BRCA mutations without progression after 4 months of chemotherapy treatment. The first-line treatments are usually selected after an oncology evaluation, grounded mostly on clinical criteria (e.g., performance status, liver function) and patients’ comorbidities. The second-line therapy selection usually considers the previous first-line protocols and patients’ performance status. During these therapeutic decisions, less than 15% of physicians consider patients preferences (see Table 2). According to the oncologists, FOLFIRINOX/mFOLFIRINOX is used as the first-line therapy in 45.0% of patients [IQR 30-50], followed by gemcitabine plus nab-paclitaxel (32.5% [IQR 20-40]). This last regimen is also commonly used as the second-line therapy (35.0% [IQR 10-50]) followed by gemcitabine alone (25.0% [IQR 10-40]) (Table 3).
Discussion
This study was triggered by the ongoing debate on the need for improving pancreatic cancer diagnosis and reducing the burden of this disease caused by the high rates of morbidity and mortality in Portugal. Through a nationwide survey with physicians that routinely manage mPDAC patients (i.e., pathologists and oncologists) from all Portuguese regions, we were able to identify their perception on some barriers for rapid diagnosis and beginning of treatment - including heterogeneous practices related to criteria for requesting molecular tests and delays for obtaining BRCA results that may impact on clinical and economic outcomes.
According to the Global Cancer Observatory, in 2020 the estimated crude incidence and prevalence (5-years) rates for PDAC in Portugal were of 17.6 and 11.7 per 100,000 habitants, respectively, with a mortality rate (2020) of 5.9 per 100,000 habitants [1]. In our study, we found pancreatic adenocarcinoma as the most reported type of tumor among patients, with around 30 cases per institution per year in Portugal, of which around 75%diagnosed in a metastatic stage or in progression, with a large dispersion (varying from 8 to 70 patients/institution/year). According to the Portuguese National Health System (SNS), the country currently has around 55,000 registered physicians, of which 30,000 work on public health institutions, especially in Lisbon and North regions that concentrate approximately 60% of all healthcare professionals [29]. These figures highlight some geo-graphical asymmetries that are also reflected in our study.
The goal of rapid investigation and treatment of cancer is to maximize cure rate for patients with early-stage disease, to increase the number of patients with resectable disease, and to avoid tumor growth and upstaging [24]. We found that physicians perform upper endoscopic ultrasound with cytology, biopsy procedures, and metastasis investigation in around half of patients admitted in their institution as part of the histological diagnosis. Complementary procedures such as PET-CT and CA 19.9 are fairly used. Although ERCP is not mandatory for pancreatic cancer (i.e., therapeutic procedure indicated in case of obstructive jaundice), it was frequently mentioned by the physicians as a complementary requested procedure for diagnosis. These differences may occur due to the limited access to these techniques or availability of resources in each center, together with the current local protocols for clinical practice. Nonetheless, precise staging of PDAC, including TNM staging and determination of tumor resectability is highly recommended by international guidelines and should be always performed [24, 30]. In the last few decades, the importance of EUS-FNB significantly increased worldwide, as it represents a step forward to a more accurate diagnosis and, consequently, to a more frequent use of neoadjuvant chemotherapy and personalized medicine. This approach has surpassed percutaneous sampling techniques, as it provides tissue core biopsies, allowing histological assessment. New generation FNB needles demonstrated a diagnostic accuracy of over 95% for solid pancreatic lesions and provide samples appropriate for ancillary testing, such as immunohistochemistry and tumor molecular pro-filing [25, 31].
We also verify the need to enlarge molecular characterization in patients with mPDAC, as only around 40% of physicians request the assessment of additional biomarkers besides BRCA1 and BRCA2. These data are in consonance with previous literature stating that although clinically relevant subtypes of mPDAC exist, molecular profiling is not yet standard in clinical care. Nonetheless, several groups and international cancer networks now advocate for universal multigene germline testing for all patients, irrespective of family history or age at diagnosis [24, 30]. The current challenges for expanding molecular analyses and precision medicine for mPDAC include, among others, the heterogeneous cellular composition of biopsy specimens, the low neoplastic cellularity of tumors and rapid progression of the disease and decisions related to clinical practice and available resources (e.g., human and technical resources, access to drug therapies toward different mutations’ treatment) [32, 33]. Interestingly, a study implementing a biopsy protocol to perform time-sensitive whole-exome sequencing and RNA sequencing for mPDAC showed that therapeutically relevant genomic alterations were identified in 48% of patients and pathogenic/likely pathogenic germline alterations in 18%.
This results, promoted in around one-third of patients a change in clinical management as a result of genomic data. The most important alterations found were germline or somatic alterations on DNA-damage repair genes (in 40%) and almost 3% had oncogenic in-frame BRAF deletions, which could confer sensitivity to MAPK pathway inhibition. Besides, the aforementioned technical issues, the clinical application of a molecular pro-filing in mPDAC patients should also consider the difficulty to propose non-approved drugs in this setting, especially considering the high costs and the doubts in response associated with those drugs. These results additionally highlight the difficulties in implementing these protocols in real-world settings [34].
Another dilemma in mPDAC management in Portugal refers to the discrepancies between pathologists and oncologists perceived clinical routines, especially regarding time to mutations’ profiling requests and results. This may occur as most pathologists usually require BRCA mutation characterization earlier-during the histological diagnosis, and receive the results within 6 weeks. On the other hand, most oncologists refer to this procedure only during the oncologic consultation in their centers, with half of them obtaining the results after 6 weeks. In this scenario, the pointed median time from patients’ admission until mPDAC diagnosis may be longer than 2 months. Comparatively, in England, the median time from patients’ first presentation to the healthcare system to diagnosis is of 76 days (IQR 28-161) for metastatic pancreatic cancer, which represents around 2-3 months [35]. In Italy, the overall median diagnostic delay for PDAC is of 2 months, varying from 1 to 5 months [36]. Regarding only the mutation profiling process for advanced cancers, in the USA, the median time to transmission of results to patients after testing is of around 1 month, but with a very large dispersion (ranging from 0 to 16 months) [37].
These findings highlight the need to standardize and accelerate disease diagnosis and staging aiming at reducing the times for decision-making regarding patients’ treatment. In an international level, both germline and tumor BRCA mutational analyses are being increasingly used for selecting patients who could benefit from PARP inhibitors. These tests should be requested during the initial diagnosis of the patient, thus providing appropriate information on all aspects associated with the disease and allowing prompt actions for managing mPDAC [38].
One approach to enhance clinical practice homogeneity and reduce time for cancer diagnosis and treatment is by implementing functional multidisciplinary (MDT) teams’ meetings and referral centers [39]. However, several factors influencing presentation of all pancreatic cancer patients to MDT meetings still exist. We found that clinical specialties such as general surgery, pathology, gastroenterology, and radiotherapy - that are paramount for PDAC management, participate in only around one-third of the so-called MDT consultations. The rationale for MDT is to be multidimensional, aiming at ensuring that complex patients receive all care services, including timely diagnosis and treatment, to meet their individual needs. These teams should bring together the expertise and skills of different professionals to assess, plan, and manage care jointly [40]. A recent study performed in Australia showed that barriers influencing MDT practices include: absence of palliative care representation, the number of MDT meetings, the cumulative cost of staff time, the lack of capacity to discuss all patients within the allotted time and reduced confidence to participate in discussions [41]. These factors can lead to a reduced quality of care management and failure to reach decisions in around 27-52%of cases [42]. Additionally, a systematic review showed that MDT decisions frequently lack on considering nursing personnel opinions and patients preferences [42]. In our study, although most oncologists feel satisfied with the support provided by different hospital services, including nursing staff and pain units, there is room for improvement in coordination with the nutrition and palliative care units. This is important as a study recently demonstrated that around 93% of patients with PDAC need palliative care referral, 45%receive palliative chemotherapy and around 80% have a dietitian referral [43]. In this scenario, key enablers influencing MDT practices include a strong organizational focus (e.g., leadership, training) that should be strengthened with the development of agreed evidence-based protocols and referral pathways, use of tech-nology (e.g., videoconferences), resource allocation and capabilities, and a culture that fosters widespread collaboration for all stages of PDAC (e.g., motivation to provide good quality care) [41, 42].
According to surveyed oncologists, the first-line treatments for mPDAC in Portugal are currently selected grounded on clinical criteria (e.g., performance status, liver function) and patients’ comorbidities, being chemotherapy combinations such as FOLFIR-INOX or mFOLFIRINOX or gemcitabine plus nab-paclitaxel the most prescribed. Selection of the second-line therapies follows similar patterns, being grounded on the use of previous chemotherapy protocols and patients’ performance status. Factors such as the access to therapies (e.g., regulatory issues, costs, reimbursement criteria) and real-world practices in the country (e.g., delayed or lack of molecular profiling, inflexible treatment protocols, physicians’ preferences) can be associated with this heterogenous scenario. This also highlights the difficulties for approving and implementing new target drugs, such as olaparib, into daily practice, which could support answering the needs of both patients and healthcare professionals in the country. Yet, around 70% of oncologists in our study stated that PARP inhibitors, when approved, would be the therapeutic choice for patients with BRCA mutations without progression after 4 months of chemotherapy treatment.
Our study has some limitations. Non-probabilistic convenience sampling in cross-sectional studies may carry out a bias in data collection and due to under-representation of subgroups considering that more committed responders usually get involved in mPDAC care. However, our inferences were grounded on the results obtained with this sample, without further extrapolation. We also acknowledged the relatively small sample size with limited number of participants reported by physicians from some regions of the country. Yet, this geographical asymmetry is similar to that observed in the country in previous studies [2]. We were able to portray the perception of both pathologists and oncologists that routinely manage mPDAC patients in Portugal. Although the questionnaire was applied in the end of 2020, which could raise concerns about the impact of the COVID-19 on the clinical activities evaluated in this study, all questions were retrospective regarding the routinely scenario previous to the pandemic.
Portuguese physicians support the increasing role of target therapies and patient-tailored treatments for mPDAC, whose selection should be grounded on tumoral subtyping and molecular profiling by means of accurate diagnostic and staging techniques. However, further efforts from both healthcare institutions and the health system to develop functional multidisciplinary teams and provide technical and qualified human resources are required. This may reduce the time between patients’ diagnosis and beginning of treatment and standardize daily clinical practice in the country. Additionally, there is a need to optimize the approval and implementation process of new target therapies for conditions such as mPDAC that would benefit from the availability of further therapeutic strategies.