Background
Schwannomas are benign encapsulated nerve sheath tumors, usually attached to peripheral nerves and arise from differentiated Schwann cells. Mostly, they are sporadic, while some are associated with syndromes such as neurofibromatosis type 2, schwannomatosis, or Carney’s complex [1]. These are spindle cell tumors that generally occur in the upper limbs, head, and neck, followed by the trunk and flexor surfaces of the lower extremities. Approximately 0.2% of all gastrointestinal (GI) tumors are constituted by schwannomas [2]. The commonest site is the stomach followed by the colon, cecum, and rectum and rarely the jejunum [3]. GI schwannomas are uncommon and usually occur in sixth to seventh decade [4]. Herein, we report a rare case of schwannoma of the common bile duct (CBD) presenting as a porta hepatis mass. Few case reports of porta hepatis schwannomas arising from CBD, hepatoduodenal liga-ment, hepatic vein, or artery have been published in the literature.
Case Presentation
A 40-year-old female, presented with the chief complaint of pain in the abdomen for the past 10 months. Pain was located in the right upper quadrant, dull in nature with no aggravating factors. There was no history of fever, jaundice, vomiting, and upper or lower GI bleeding. The patient did not report any altered bowel habits, loss of appetite, or weight. Patient had no medical comorbidities. The general physical and abdominal examination was unremarkable. Complete hemogram and renal function tests were within normal limits. SGOT and SGPT were 26 and 25 IU/L, respectively. Serum bilirubin was 1.2 mg% while alkaline phosphatase was 186 IU/L.
Ultrasonography (USG) of the abdomen showed a hypoechoic mass at the porta abutting the right portal vein and the main portal vein (shown in Fig. 1). The liver was normal with no intrahepatic biliary radicle dilation and showed a normal echotexture. No ascites was reported. Contrast-enhanced computed tomography abdomen revealed a mass at the porta extending from the portal bifurcation till the hilum encasing the main portal vein and abutting the right portal vein (shown in Fig. 1). Common hepatic artery was free. Contrast-enhanced magnetic resonance imaging (MRI) abdomen showed a mass at the porta abutting the right portal vein, main portal vein, right hepatic artery, and the common hepatic artery (shown in Fig. 1). The mass was extending between the head of the pancreas and the inferior vena cava. Vertically, the mass was extending between the hilum and retropancreatic region. Endoscopic ultrasound was also done which revealed a mass from the superior mesenteric vein/portal vein confluence till the hilum (shown in Fig. 2). Fat planes with the main portal vein were maintained. Based on overall clinical and imaging findings, possibilities of carcinoma, lymphoma, and mesenchymal tumor were considered.
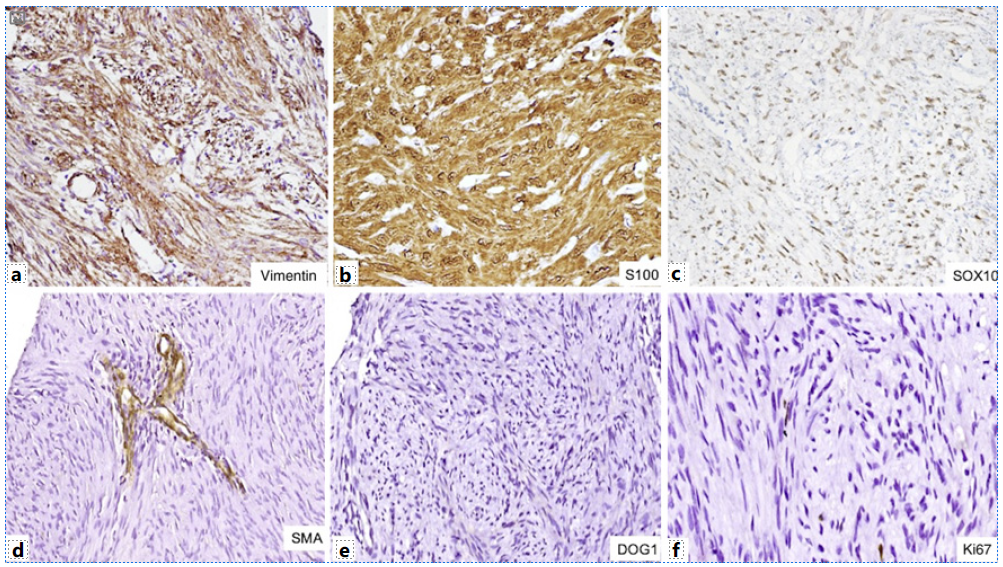
USG-guided biopsy was performed on the mass which showed a benign nerve sheath tumor, immunopositive for S100 (shown in Fig. 3). Following the above investigations, the patient was undertaken for surgery. En bloc excision of the mass and CBD with Roux-en-Y hepatico-jejunostomy was performed. The resection specimen was submitted to the department of pathology. Gross examination showed an encapsulated, circumscribed, yellow-white firm tumor measuring 7.5 cm in maximum dimension (shown in Fig. 3). On microscopy, it was a biphasic tumor composed of hypercellular areas with fascicular arrangement of spindle cells and palisades (Verocay bodies) along with myxoid hypocellular areas and focal hyalinization. The spindle-shaped tumor cells contained moderately ill-defined cytoplasm, wavy tapering nuclei with fine granular nuclear chromatin, and inconspicuous nucleoli. In addition, there were many interspersed blood vessels with hyalinized walls along with lymphoid infiltrate at the tumor periphery (shown in Fig. 3). No significant nuclear pleomorphism, mitosis, or necrosis was noted. On immunohistochemistry, the tumor cells were immunopositive for vimentin, S100, and SOX10 while they were negative for CK, CD34, smooth muscle actin, CD117, DOG1, myogenin, ALK, STAT6, CD21, and HMB45. Ki67 proliferation index was less than 1% (shown in Fig. 4). Based on the immunohistomorphological profile, the tumor was diagnosed as schwannoma. Patient recovered well postoperatively and was discharged on postoperative day 10. The patient is disease-free on follow-up after 36 months.
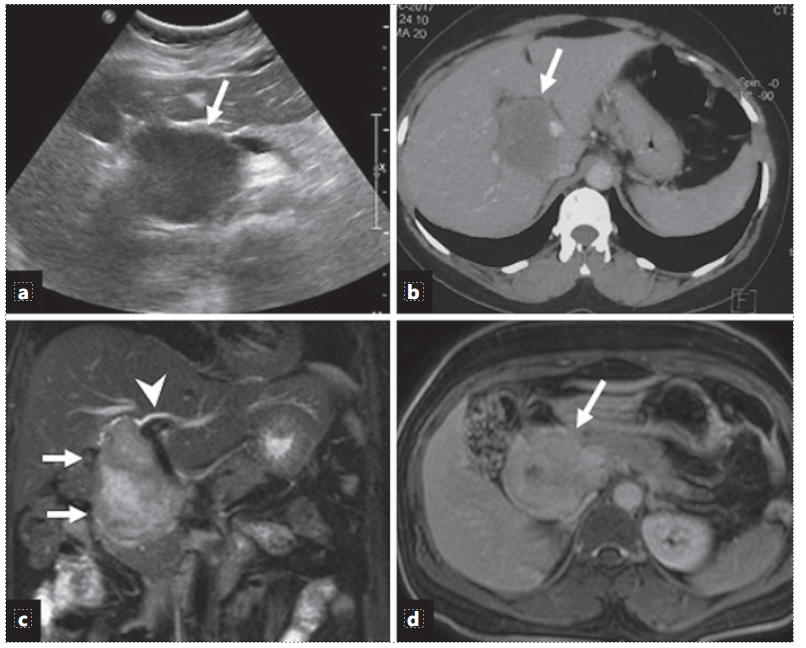
Fig. 1 a Ultrasound image shows a hypoechoic mass (arrow) at the porta hepatis. b Axial contrast-enhanced CT image shows a hypodense mass (arrow) at the porta hepatic splaying the portal veins. c Coronal T2-weighted MR image shows an oblong hyperintense mass (arrows) along the course of the CBD with mild intrahepatic bile duct dilatation (arrow-head). d Axial contrast-enhanced T1-weighted MR image (5 min delayed) shows late enhancement of the mass (arrow).
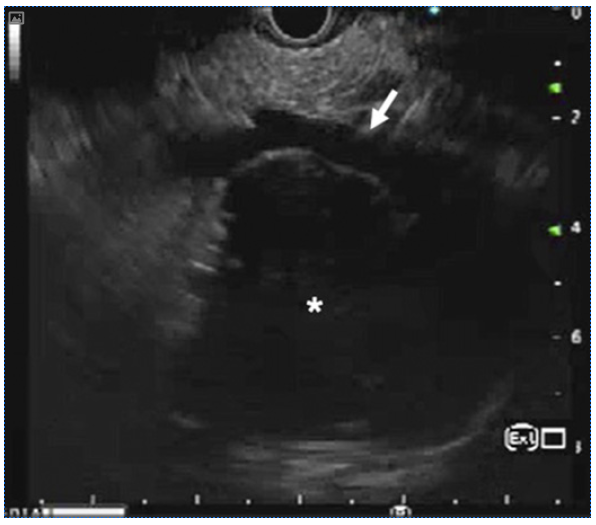
Fig. 2 Endoscopic ultrasound shows a hypoechoic mass (asterisk) at the porta hepatis abutting the portal vein (arrow). Image courtesy of: Dr Deepak Gunjan.
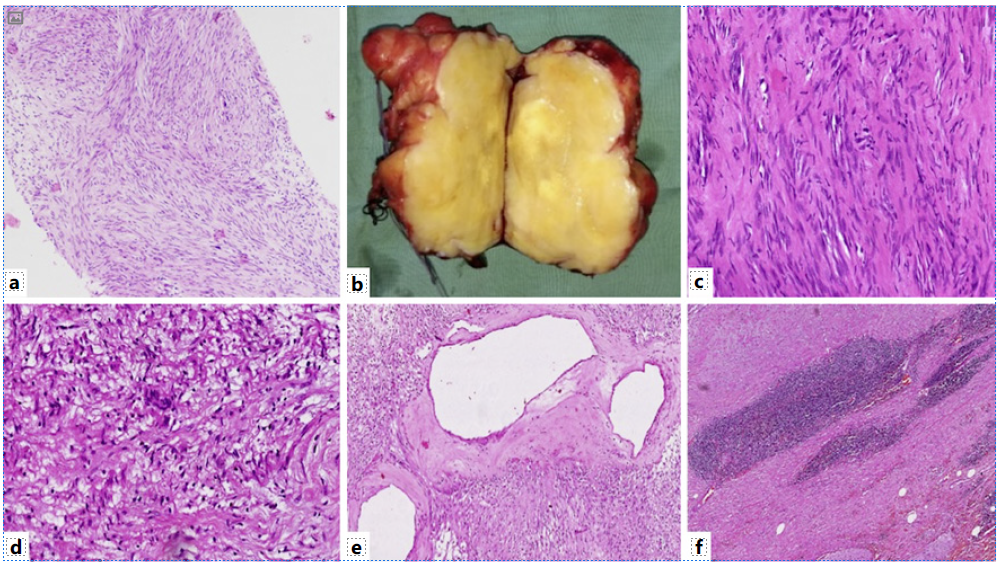
Fig. 3 a Biopsy section shows a benign spindle cell tumor (×100, H&E). b Gross examination shows nodular circumscribed yellow-white firm tumor. The tumor shows hypercellular areas with Verocay bodies (c) (×400, H&E) and hypocellular areas (d) (×400, H&E). Also, many dilated vessels with perivascular hyalinization (e) (×200, H&E) and lymphoid cuff at tumor periphery (f) (×200, H&E) are seen.
Discussion
Schwannomas in porta hepatis and biliary tree are very rare and approximately 30 cases have been reported in literature till date. There are 22 reported cases of schwannoma in the biliary tract alone details of which have been summarized in Table 1 [3-24].
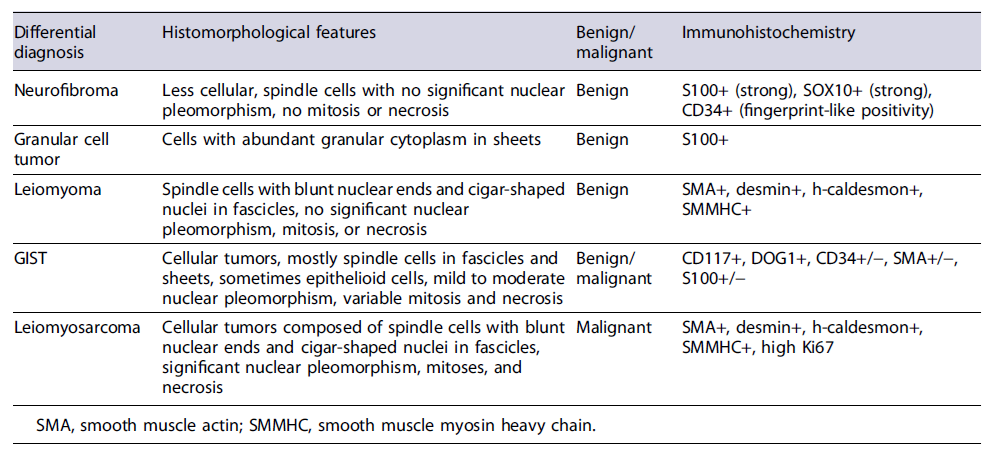
A significant female predominance with male-to-female ratio of 1:5.5 was seen in these patients. The mean age at presentation was 48.3 years (range 15-78 years). Abdominal pain and jaundice were the most common presenting symptoms in these patients (jaundice in 38%, abdominal pain in 19%, both pain and jaundice in 23% of patients, respectively). The most common location was CBD (57%). The preoperative clinico-radiological diagnoses were quite variable and comprised metastatic melanoma, gastrointestinal stromal tumor (GIST), lymphoma, adenocarcinoma, leiomyosarcoma. In all the cases, it was extremely difficult to correctly diagnose this tumor preoperatively, mainly due to the fact that tumors at this site can easily mimic bile duct adenocarcinoma and other malignancies such as lymphoma and IgG4-related diseases. Moreover, the location is difficult to approach for a minimally invasive technique such as fine needle aspirate. Surgical resection was carried out in most cases with unremarkable postoperative period [3-24]. In our case, a correct preoperative diagnosis on USG-guided biopsy helped in the adequate surgical management. Radiologically, contrast-enhanced MRI is better suited to visualize the extent and size of such soft tissue tumors and dilatation of bile duct radicles due to mass effect [25]. On MRI, schwannomas are hypointense on T1-weighted images and homogeneously hyperintense on T2-weighted images [26]. Usually, degenerative changes are uncommon in GI schwannomas, but if present can lead to error in diagnosis. Diagnosis in such cases can only be made on histology which requires excision of the mass. The most common differential diagnoses for schwannoma are other benign and malignant soft tissue tumors of the gastrointestinal tract such as neurofibroma, leiomyoma, GIST, and leiomyosarcoma, enumerated in Table 2.
Table 2 Discussion of common differential diagnoses of soft tissue masses in the biliary tract region
Histologically, schwannomas show hypercellular areas (Antoni A) with Verocay bodies and hypocellular (Antoni B) areas. The tumor cells are predominantly spindle-shaped with hyperchromatic nuclei. Mitosis and necrosis are usually absent [10]. Schwannomas can show an array of degenerative changes such as hyalinization, calcification, emorrhage, myxoid change, cyst formation, focal bizarre nuclear atypia. Multiple variants are seen, namely, ancient, plexiform, cellular, epithelioid, microcystic or reticular, and melanotic or pigmented [14].
Immunohistochemistry plays an important role in distinguishing schwannoma from close differentials such as GIST, leiomyomas, or neurofibromas. Schwannomas are strongly immunopositive for S100 protein and SOX10. GISTs are immunopositive for c-KIT and DOG1, while leiomyoma and leiomyosarcoma will show strong and diffuse smooth muscle actin positivity. CD34 and calretinin might help in differentiating these from neurofibroma as both are immunopositive for S100. Neurofibroma shows moderate CD34 positivity. Fine et al. [27] in their study compared calretinin positivity in schwannoma and neurofibroma and 96% of schwannomas displayed strong calretinin staining compared to only 7% in neurofibroma. GI schwannomas show some histomorphological variations when compared to schwannomas at other regions in the form of predominance of hypercellular areas (Antoni A) and lack of nuclear palisading pattern as seen in conventional schwannoma [28]. Our case, although it showed both the areas, Lasota et al. [29] also reported lack of NF-2 gene alterations in the GI schwannomas suggesting it to be a morphologically and genetically distinct group of nerve sheath tumors. However, more data needs to be incorporated to arrive successfully at this conclusion. Being benign tumors, these have an excellent prognosis and complete surgical resection is the mainstay of treatment. Recurrence or malignant transformation is rarely seen [30].