Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the novel coronavirus disease 2019 (COVID-19), first reported in December 2019 in Wuhan, China, and later becoming a global pandemic [1]. Since the onset of the COVID-19 pandemic, major efforts were made in the development of a SARS-CoV-2 vaccine, leading to the fast development of safe and highly effective vaccines.
The immunological response to vaccines leads to the release of inflammatory cytokines, antibodies targeted to the virus’ spike protein, blocking its entrance in host cells, and the activation of Th1 lymphocytes, leading to the expansion of CD4+ and CD8+ T cells and the development of memory T cells [2-4]. The primary immunological endpoint of COVID-19 vaccines was the induction of neutralizing antibodies against the SARS-CoV-2 spike protein [4], which correlate with binding anti-Spike antibody titers and are detected in 90% of the seroconverters [5]. Clinical endpoints of vaccine efficacy trials were assessed through virologically confirmed symptomatic SARS-CoV-2 infection and virologically confirmed SARS-CoV-2 infection with symptoms classified as severe [6].
The Pfizer/Comirnaty® [BNT162b2 mRNA], Moderna/Spikevax® [mRNA-1273], and AstraZeneca/Vaxzevria® [ChAdOx1-nCoV-19] vaccines showed excellent safety profiles and good efficacy in preventing symptomatic COVID-19 (62-95%) in the general population, but data were lacking regarding their effect in certain types of patients in clinical trials, such as those with chronic liver disease (CLD)[7]. Patients with CLD were excluded from the ChAdOx1-nCoV-19 trials and were not specifically identified in trials from the other two mRNA vaccines. Also, individuals under chronic immunosuppressive treatment such as autoimmune hepatitis (AIH) patients or liver transplant recipients were excluded from all the trials [7]. Similarly, in Janssen/Jcovden® [Ad26.COV2.S] trials, only healthy individuals or a very small proportion of patients with CLD were included [8].
The innate and adaptive immune system responses are dysregulated in patients with CLD and may be further worsened by the use of immunosuppressant drugs in AIH [9, 10]. Due to the greater incidence and severity of infections in patients with liver cirrhosis (LC), vaccination against influenza, Streptococcus pneumoniae, hepatitis A virus, and hepatitis B virus is recommended. However, the durability of humoral immunity after influenza and pneumococcal vaccination is reduced [7], and these patients have lower rates of seroconversion in hepatitis A virus and hepatitis B virus vaccination [9]. Similarly, in the case of SARS-CoV-2 infection, patients with liver disease were expected to have an attenuated response to vaccination against COVID-19 [9, 10]. This response could be further affected by the cause and staging of cirrhosis, the use of certain medications such as immunosuppressants and patients’ comorbidities. In addition, concerns regarding adverse reactions to COVID-19 vaccination in this vulnerable population, such as the risk of vaccine-triggered immune-mediated hepatitis and vaccine-induced thrombotic thrombocytopenia, were raised [11].
Furthermore, patients with LC who develop SARS-CoV-2 infection are associated with a more severe course of disease with worse clinical outcomes, when compared to non-cirrhotic patients [12, 13], making this susceptible population a priority for immunization and raising concerns regarding these patients’ adequate protection. SARS-CoV-2 infection was associated with cirrhosis decompensation in 46% of the cases, with greater rates of decompensation, hospitalization, and mortality in patients with more advanced liver disease [12].
To conclude, previously available trials and real-life data regarding immunological and clinical efficacy of COVID-19 vaccination in patients with LC were scarce, raising concerns regarding their possibly lower immunological response to vaccines and worse clinical outcomes. Previously published data on these topics have shown conflicting results. While similar seroconversion rates have been described [14], other studies reported lower antibody titers in LC patients [15, 16]. Also, some authors have found similar infection rates after vaccination [17], while others saw a delayed reduction on the incidence of SARS-CoV-2 infection after vaccination between patients with and without LC [18]. Although most of the general population is now immunized either through vaccination or infection, the risk of spreading of new and more pathogenic variants can become a significant burden to healthcare systems and have particularly harmful consequences in vulnerable populations like LC patients. Our goals were to evaluate the safety, immunological, and clinical responses of patients with LC to COVID-19 vaccination and assess group differences regarding demographic, clinical, and vaccine-related factors.
Methods
Study Design and Patient Selection
We conducted a multicentric observational prospective study in adult patients with LC regularly followed in one of six hospitals in Portugal, eligible for vaccination against COVID-19 at the time of enrollment, with a 12-month follow-up after vaccination. Follow-up would be interrupted earlier in case of an additional booster dose of the vaccine, SARS-CoV-2 infection, or death. The introduction of a universal booster dose in our country during the study period led to follow-up termination before the 12-month timepoint in all patients.
Patients eligible for the study were adult subjects with a diagnosis of LC either confirmed by liver biopsy or through unequivocal clinical, biochemical, radiological, transient elastography, and/or endoscopic features of cirrhosis. Exclusion criteria were (1) contraindications for the COVID-19 vaccination program, (2) full vaccination before recruitment,(3) previously documented COVID-19 infection (either through a positive SARS-CoV-2 nucleic acid amplification test, rapid antigen test, or antibody measurement), (4) human immunodeficiency virus infection, and (5) treatment with immunosuppressant drugs for conditions other than AIH, to minimize confounding related to immunosuppression and lower vaccine responses [19].
All the patients enrolled in the study provided informed consent, in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committees of all the hospitals involved.
Patient Assessment and Follow-Up
Patients were assessed for eligibility on a regular hospital observation in six different hospitals in Portugal between May and August 2021. If they agreed to participate, the physician filled in a questionnaire with their baseline characteristics at the time of recruitment, including demographic, clinical, and complementary diagnostic test information. Patients were described regarding their age, gender, cause of LC, previous history of cirrhosis decompensation, features of portal hypertension, Child-Pugh-Turcotte (CPT) and Model for End-stage Liver Disease (MELD-Na) scores, important comorbidities, and current medication.
Patients were followed until completing COVID-19 vaccination (defined by the administration of 2 doses of the Pfizer/Comirnaty® [BNT162b2], AstraZeneca/Vaxzevria® [ChAdOx1nCoV-19], or Moderna/Spikevax® [mRNA-1273] vaccines or one dose of the Janssen/Jcovden® [Ad26.COV2.S] vaccine) through scheduled appointments, calls, or by regularly consulting the national healthcare data platform, where information regarding the timing and type of vaccine administered was provided. By this time, the vaccination date and type were recorded, and disease staging was reassessed and updated. Afterward, either by scheduled appointments or phone calls, patients were assessed at different timepoints: 2 weeks, 3 months, 6 months, and 12 months after completing vaccination, and/or until being given a booster dose of the vaccine, developing SARS-CoV-2 infection, or death.
Our main goals were to assess the safety and immunological and clinical efficacy of COVID-19 vaccination in patients with LC. Safety was ascertained through patients’ self-reported data on adverse events following vaccination. Efficacy was measured both through humoral response (induction of immunoglobulin G [IgG]-binding antibodies against the SARS-CoV-2 spike protein) and clinical response (virologically confirmed SARS-CoV-2 infection and its severity). The severity of SARS-CoV-2 infection was defined according to the World Health Organization (WHO) Living Guidance [20] into mild (symptomatic disease without evidence of viral pneumonia or hypoxia), moderate (clinical signs of pneumonia but oxygen saturation [SpO2] ≥90% on room air), severe (clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min, severe respiratory distress, or SpO2 <90% on room air), and critical (acute respiratory distress syndrome).
Patients were given a pseudoanonymized code and instructed to go to a partner laboratory to collect blood samples for an in vitro chemiluminescent immunoassay to quantify spike-protein IgG SARS-CoV-2 antibody titers developed 2 weeks, 3 months, and 6 months after completing vaccination. The first timing for antibody measurement was decided based on clinical trials’ results for different COVID-19 vaccines, most of which had shown the greatest antibody titers 2 weeks after completing vaccination [21-24]. Antibody titers were reported in the WHO international standard binding antibody units (BAU)/mL [25]. The blood samples were destroyed after the laboratory result was released. In every timepoint, participants were also asked about the development of symptomatic SARS-CoV-2 in-fection and its severity, namely, the need for hospitalization and admission to an intensive care unit.
Our secondary aim was to assess differences between groups regarding seronegative responses, suboptimal antibody titers, and SARS-CoV-2 infection, using subgroup analysis considering their age, gender, cause and severity of cirrhosis, use of immunosuppressant drugs, relevant comorbidities, and type of vaccine given. To compare patients in terms of humoral response, two cutoffs for spike-protein SARS-CoV-2 IgG antibody titers were defined: titers under 33.8 BAU/mL were classified as a seronegative result (the 33.8 BAU/mL was the threshold for positivity, as determined by the laboratory performing the anti body measurements [Centro de Medicina Laboratorial Germano de Sousa]), and titers under 200 BAU/mL were subjectively considered suboptimal levels (defined upon results from one of the first studies of vaccine immunogenicity in CLD patients [26]).
Statistical Analysis
Statistical analysis was performed using Stata®17 (StataCorp, College Station, TX, USA). Continuous variables were described by their mean ± standard deviation (SD) or median and interquartile range [Q1; Q3], according to the observed distribution of the variables (normal or other, respectively), along with their minimum (min) and maximum (max) values when appropriate. Categorical variables were presented by observed absolute (n) and relative (%) frequencies. The comparison of antibody titers between two groups was made using Student’s t test for independent samples for normally distributed data and the Mann-Whitney U test for data without normal distribution. The association between SARS-CoV-2 infection and qualitative variables was assessed using the χ2 test. Group differences in terms of seronegative, suboptimal antibody responses, and SARS-CoV-2 infection were described and assessed in a multivariable logistic regression model through a stepwise approach to fitthemodelandfind independent clinical predictors at baseline considering the patients’ characteristics and type of vaccine administrated, with reported odds ratios and 95% confidence intervals whenever appropriate. A p value <0.05 was considered statistically significant.
Results
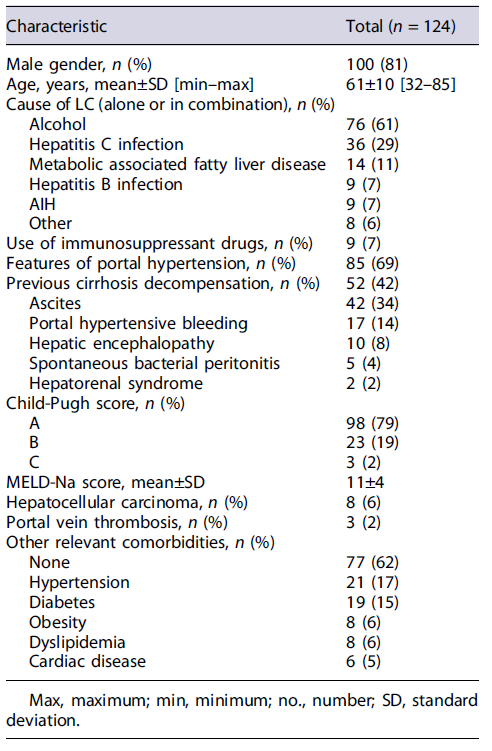
We included 124 patients in the study, 81% males, mean aged 61 ± 10 years. Alcohol was the most common cause of LC (61%). Nine (7%) patients were under immunosuppressant drugs for AIH. Sixty-nine percent of the patients had features of portal hypertension, 42% had a previous cirrhosis decompensation, and 21% had a CPT score of B/C. Table 1 outlines patients’ baseline characteristics.
All the patients were fully vaccinated between May and August 2021. The type of vaccine administrated was one of the four approved in Portugal at that time: Pfizer/Comirnaty® [BNT162b2] (n = 59, 48%), AstraZeneca/Vaxzevria® [ChAdOx1nCoV-19] (n = 45, 36%), Moderna/Spikevax® [mRNA-1273] (n = 14, 11%), and Janssen/Jcovden® [Ad26.COV2.S] (n = 6, 5%). They were followed during a mean period of 221 ± 26 days, until the timing of the booster dose administration, SARS-CoV-2 infection, or death (shown in Fig. 1). The 6-month analysis excluded 67 patients who had an earlier booster dose, 7 who were infected with SARS-CoV-2, and 2 who died of unrelated causes to SARS-CoV-2 infection. During follow-up, there was no progression or newly-onset complications associated with the patients’ underlying liver condition.
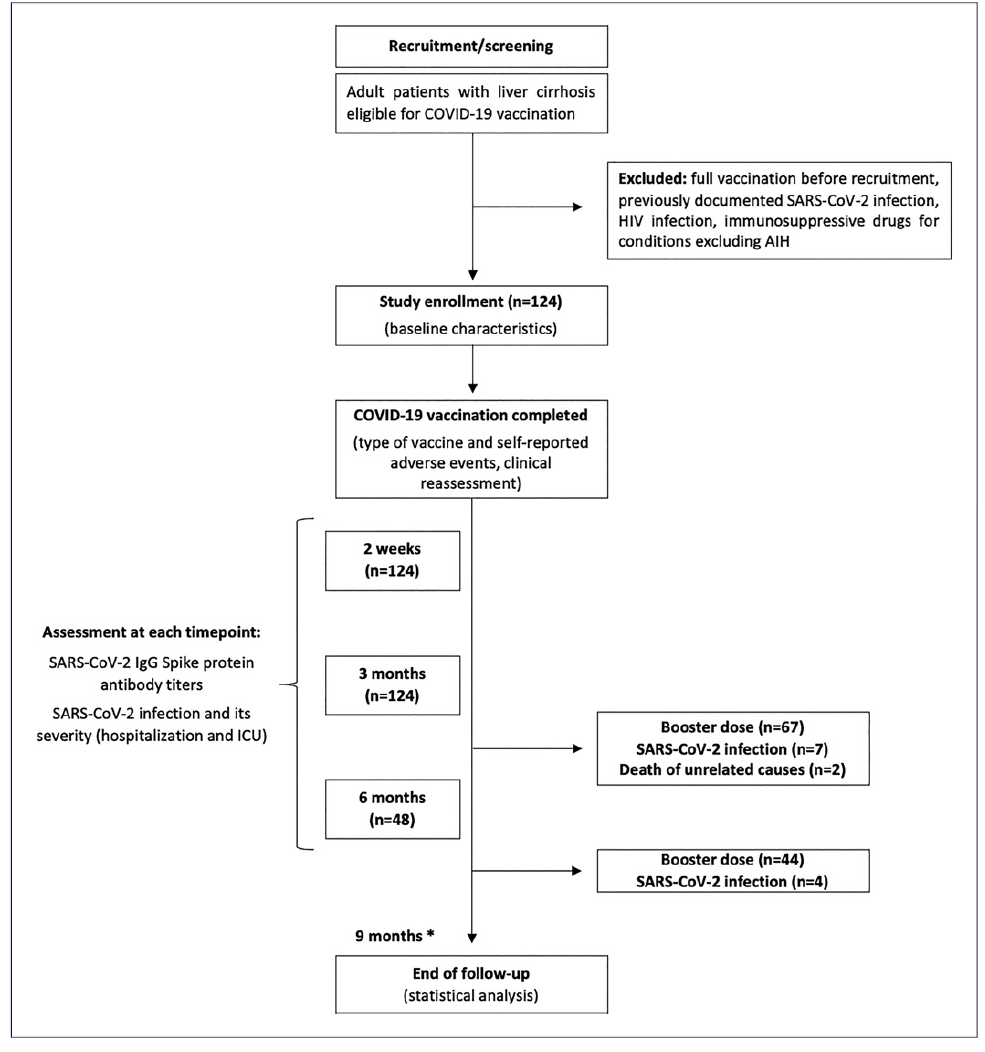
Fig. 1 Flowchart of patient enrollment and follow-up. *The end of follow-up by 9 months was related to the timing of the last booster dose given. AIH, autoimmune hepatitis; HIV, human immunodeficiency virus; ICU, intensive care unit; IgG, immunoglobulin G.
Eighteen percent of the patients (n = 22) reported vaccine-related adverse events, none of them serious. These complaints included fever (6%), myalgia (6%), fatigue (5%), diarrhea (2%), and headache (1%). They were all self-limited and did not make them seek medical care.
As for humoral response, the median spike-protein IgG SARS-CoV-2 antibody titers were 1,185 [280; 2,080] BAU/mL at 2 weeks, 301 [72; 1,175] BAU/mL at 3 months, and 192 [49; 656] BAU/mL at 6 months after completing vaccination (shown in Fig. 2). We reported negative (<33.8 BAU/mL) and suboptimal (<200 BAU/mL) anti-body titers in 8% and 23% of the patients at 2 weeks, 16%and 38% at 3 months, and 22% and 48% at 6 months.
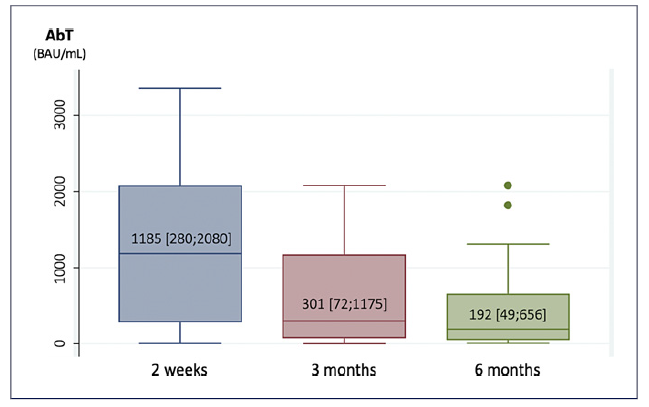
Fig. 2 Median [Q1; Q3] spike-protein IgG SARS-CoV-2 antibody titers (AbT) at 2 weeks, 3 months, and 6 months after completing vaccination.
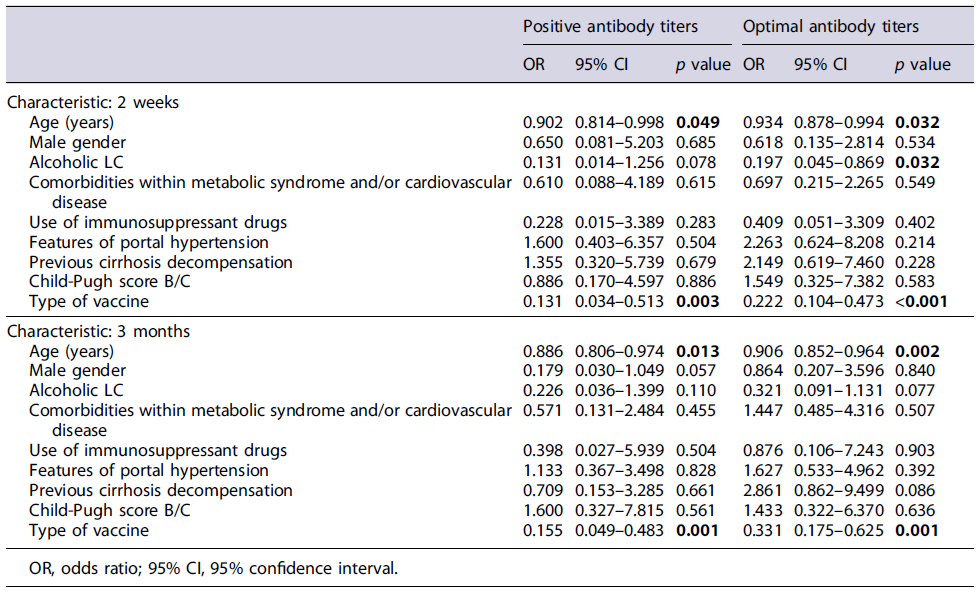
Older age was associated with negative and suboptimal antibody titers in almost every timepoint analyzed using a multivariable logistic regression model (Table 2). Patients with negative or suboptimal titers at 2 weeks were 65 ± 10 years compared to 60 ± 10 years (p = 0.049) and 64 ± 11 years versus 59 ± 10 years (p = 0.032), respectively. Similar differences were also seen at 3 months, when patients with negative or suboptimal titers were 66 ± 9 years versus 60 ± 10 years (p = 0.013) and 65 ± 8 versus 59 ± 10 years (p = 0.002), respectively, and at 6 months, with negative or suboptimal titers in patients aged 68 ± 6 years compared to 55 ± 8 years (p = 0.050) and 60 ± 9 years versus 54 ± 7 years (p = 0.084).
Table 2 Multivariable logistic regression analysis of patients’ characteristics and positive (>33.8 BAU/mL) or optimal (>200 BAU/mL) antibody titers at 2 weeks and 3 months
Alcoholic LC was associated with suboptimal antibody titers at 2 weeks (29% compared to 14% in nonalcoholic cirrhosis, p = 0.032). Considering the 9 patients with AIH under immunosuppressive drugs, 11% had negative and 22% had suboptimal antibody levels at both 2 weeks and 3 months after completing vaccination, without statistically significant differences compared to the remaining patients; only 2 patients were eligible for the 6-month analysis, and this was therefore not performed. Group differences regarding liver disease severity and antibody titers are shown in Figure 3 and were not statistically significant.
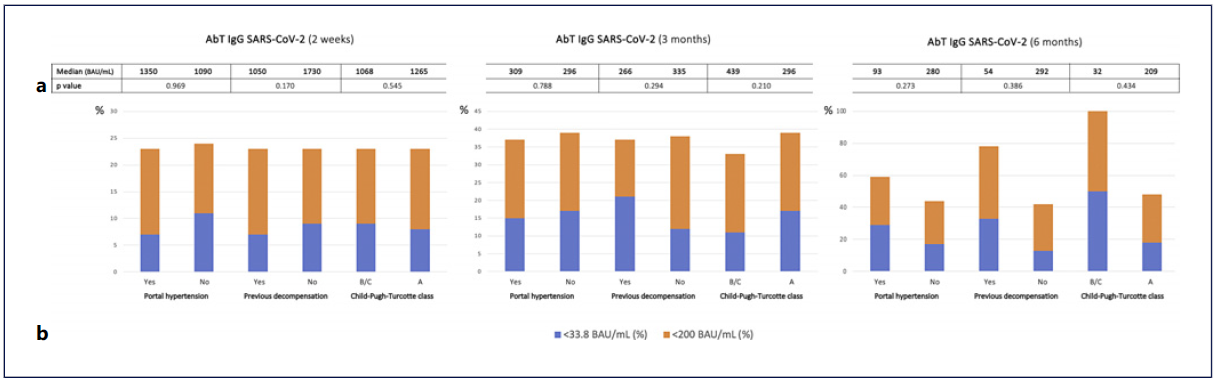
Fig. 3 Group comparisons in terms of liver disease severity regarding spike-protein IgG SARS-CoV-2 antibody titers (AbT) at 2 weeks, 3 months, and 6 months after completing vaccination. a Median AbT (BAU/mL) in each group; p values were obtained using a Mann-Whitney U test. b Proportion (%) of patients in each group with AbT less than 33.8 BAU/mL (blue) and 200 BAU/mL (orange).
The mRNA-1273 vaccine had the highest median anti-body titers throughout every timepoint of analysis, followed by the BNT162b2, as shown in Figure 4. Adenovirus vector vaccines (Ad26.COV2.S and ChAdOx1nCoV-19) were associated with lower median antibody titers when compared to mRNA vaccines, both at 2 weeks and 3 months (shown in Fig. 4; Table 2). In the 6-month analysis, there was a lower number of patients in each group; therefore, these differences were not analyzed.
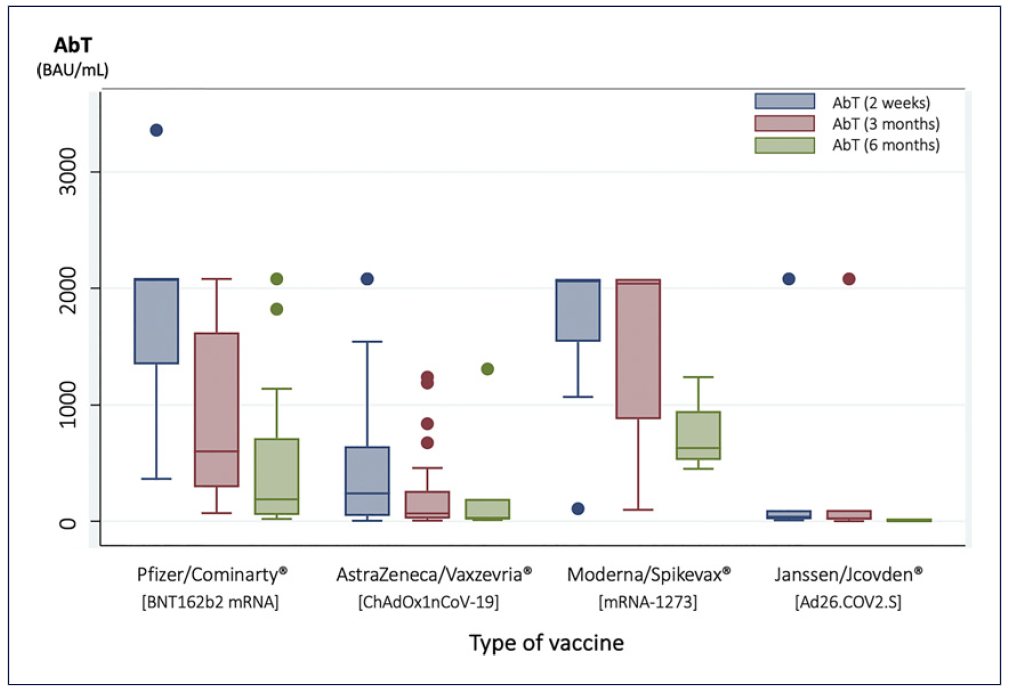
Fig. 4 Median spike-protein IgG SARS-CoV-2 antibody titers (AbT) according to type of vaccine administrated at 2 weeks, 3 months, and 6 months after completing vaccination.
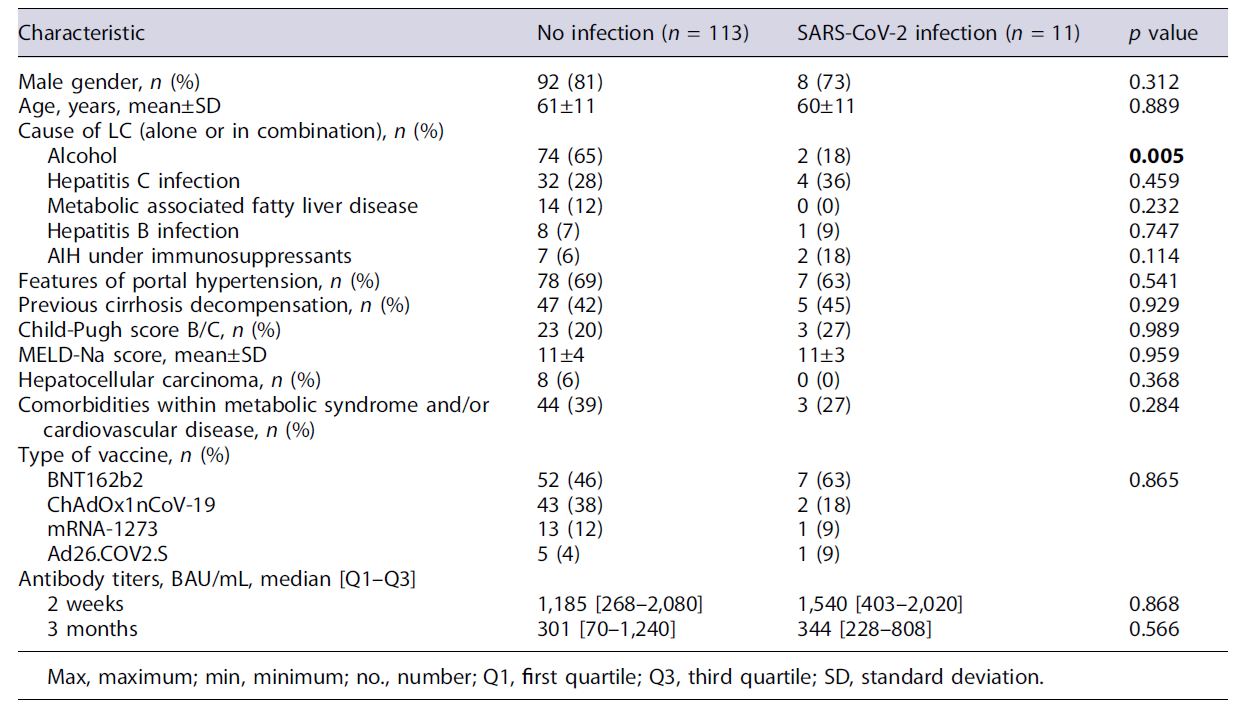
Eleven patients (9%) developed SARS-CoV-2 infection during the follow-up period, occurring 3.8-6.6 months after completing vaccination. All of them had asymptomatic (n = 1) or mild (n = 10) disease, without the need for hospitalization. They were 73% males, with a mean age of 60 ± 11 years. Except for alcoholic LC, which was associated with lower rates of SARS-CoV-2 infection (p = 0.005), we did not find other associations between patients’ characteristics and the development of SARS-CoV-2 infection (Table 3), namely, type of vaccine administrated and spike-protein SARS-CoV-2 IgG antibody titers. In fact, 8 patients (73%) had spike-protein SARS-CoV-2 IgG antibody titers greater than 200 BAU/mL on the 3-month analysis.
Discussion
Infections are a common cause of decompensation and death in patients with LC. Their dysregulated immune system makes them not only more susceptible to certain infections but also more likely to develop serious disease [27]. In a large international cohort of unvaccinated CLD patients, SARS-CoV-2 infection in patients with LC led to liver decompensation in 46% of the cases, half of them with acute-on-chronic liver failure, and a 32% mortality rate, compared to 8% in patients without cirrhosis (p < 0.001) [12].
Although nowadays much evidence regarding COVID-19 vaccination in LC patients has been published, most of the studies were based on retrospective cohorts from large databases [14, 17, 28] and did not simultaneously assess safety, humoral, and clinical responses in patients with LC.
In our study, side effects were observed in 18% of the patients, but they were all mild and consisted mostly of fever, myalgia, and fatigue, similar to the results reported from clinical trials [29]. The good safety profile of COVID-19 vaccines in LC was in line with other studies in this population [14]. A safety assessment of the ChAdOx1-nCOV vaccine [30] showed a rate of systemic adverse events of 22%, with fever being the most common symptom, and all of them were mild and transient, just like in other cohorts of patients [31].
Specific concerns of safety of COVID-19 vaccination in this population would be vaccine-triggered immune-mediated hepatitis and vaccine-induced thrombotic thrombocytopenia, resulting in splanchnic and hepatosplenic thrombosis [11]. However, immune-mediated hepatitis has been rarely reported, and no causal link to the vaccine has yet been established, to the point that the occurrence of liver injury after vaccination is not a contraindication to subsequent vaccination. Thrombotic thrombocytopenia, a rare event after COVID-19 vaccination with adenoviral vector vaccines which could lead to cirrhosis decompensation, was also not observed in our study, even though we acknowledge that our sample size was too low to detect these infrequent side effects.
In this study, we assessed immunological response through humoral response with spike-protein IgG SARS-CoV-2 antibody titer measurement at three different timepoints.
We observed a seroconversion rate of 92% in LC patients 2 weeks after vaccination. A meta-analysis on COVID-19 vaccine immunogenicity among CLD patients [14] reported a good humoral response to in-activated and mRNA COVID-19 vaccines, with total seroconversion rates of 85% either in cirrhotic or non-cirrhotic CLD patients (with pooled seroconversion rates of neutralizing and anti-spike antibodies of 84% and 92%, respectively). However, even though patients with LC seem to develop similar seroconversion rates, some studies have described lower antibody titers compared to non-cirrhotics. For example, a study by Willuweit et al.[32] reported similar seroconversion rates (96% vs. 99%, p = 0.400) after 2 doses of the BNT162b2 vaccine but lower IgG SARS-CoV-2 titers (939 vs. 1,905 BAU/mL, p = 0.0001) in patients with LC compared to controls. Importantly, the timing for antibody measurement was different between groups (median 69 vs. 56 days for cirrhotics and controls, respectively), which may have affected the results. Another study in patients vaccinated with BNT162b2 (70.3%), mRNA-1273 (18.9%), or ChAdOx1nCoV-19 (10.8%) [15] reported a nonsignificant trend to lower IgG antibody levels in patients with cirrhosis or advanced liver fibrosis (F3-F4) compared with healthy controls, 2 weeks and 6 months after vaccination.
Antibody titers decreased in subsequent evaluations during our follow-up period. This waning humoral response has been described in a study by Levin et al. [33] in healthcare workers, with the level of IgG antibodies decreasing at a consistent rate up to 6 months of follow-up after the second dose of the BNT162b2 vaccine. In a study comparing LC patients with controls, this waning was seen similarly in both groups at 6 months [15].
We did not evaluate T-cell reactivity. A decreased T-cell response could be a possible explanation for the occurrence of SARS-CoV-2 infection despite adequate antibody levels. In fact, patients with SARS-CoV-2 infection had nonsignificant greater median antibody titers than uninfected patients, which support the role of other immune mechanisms in conferring protection. A greater cellular response could explain lower rates of infection in subgroups with suboptimal antibody titers as reported for alcoholic LC. Previously published data have been inconsistent, with studies reporting T-cell responses in cirrhotic patients similar to controls [16] and others showing an impairment in T-cell reactivity in cirrhotic patients compared to controls (36% vs. 6%)[34]. These studies are limited by the different timing for antibodies’ measurement, which may have affected the results.
In our cohort of vaccinated LC patients, only 9%(ranging from 4% to 17% according to the type of vaccine) developed COVID-19 during the peak of the pandemic. None of the patients developed moderate-severe SARS-CoV-2 infection, and no hospital admission, intensive care unit admission, or death were reported. We acknowledge that the participation in this study could have led to a subject bias with potential overestimation of the clinical efficacy, with patients avoiding high-risk behaviors that would increase the likelihood of being infected with SARS-CoV-2. However, our results are in line with the ones reported in the literature, either in healthy or in CLD subjects. Indeed, clinical trials have reported a vaccine efficacy against symptomatic/moderate/severe COVID-19 of 95%, 94%, 70%, and 67% for the BNT162b2, mRNA-1273, ChAdOx1nCoV-19, and Ad26.COV2.S vaccines, respectively [35].
Similar good results were also described in previous retrospective studies in CLD [28]. A study from a large US database on 20,037 liver cirrhotic patients propensity-matched to 20,037 non-cirrhotic controls vaccinated with at least one mRNA vaccine dose [18] showed a delayed, but robust 78.6% reduction on the incidence of COVID-19 infection after the second dose, and an excellent reduction of 100% in COVID-19-related hospitalization and mortality in patients with LC. Another large cohort of 68,048 unvaccinated compared to 10,441 vaccinated CLD patients with cirrhosis [17] reported that 15% versus 3.7%developed SARS-CoV-2 infection and 15.2% versus 7.7%of these needed mechanical ventilation or died in a 30-day follow-up, respectively.
We found no correlation between antibody titers and the development of SARS-CoV-2 infection in our population, with 73% of the patients that developed SARS-CoV-2 infection presenting adequate titers 3 months after vaccination. A systematic review on the correlation of antibody levels to vaccine efficacy [36] reported that, while in some studies there was a correlation between them, in others there was an inverse relationship between antibody levels and infection incidence, risk, or viral load, suggesting that both humoral immunity and other im-mune components contribute to protection.
In our study, older age was associated with lower antibody titers, which has been described in previous studies either in patients with CLD [26] or in healthcare workers [33]. These studies also reported male gender and immunosuppression as risk factors for worse humoral responses [26, 33]. In our population, we did not see these associations, which may be explained by the small proportion of females and patients under immu-nosuppression limiting adequate group comparisons.
Despite not finding statistically significant associations, limited by the small group size, from the 9 patients under immunosuppressants, 2 (18%) developed SARS-CoV-2 infection, compared to 9 (8%) of the remaining participants. This suggests that the first may have been more susceptible to lower vaccine efficacy, although infection severity was not worse. Previous studies have reported a negative correlation between immunosuppressive treatment and anti-SARS-CoV-2 antibody titers and neutralizing activity compared to other patients with CLD [37] and a lower T-cell response of patients with AIH when compared to patients with primary biliary cholangitis or primary sclerosing cholangitis [38], even in the ones without immunosuppressive treatment. Despite these findings, clinical efficacy of SARS-CoV-2 did not seem impaired, with reports of a significant reduction on the risk of COVID-19 severity and mortality in patients with AIH [39].
In our cohort, patients with alcoholic LC were associated with suboptimal antibody responses at 3 months but a lower SARS-CoV-2 infection rate. A possible explanation would be a greater influence of the unmeasured T-cell response in preventing SARS-CoV-2 infection, as previously mentioned. Besides, our inability to assess patients’ lifestyle behaviors that could make them more susceptible to SARS-CoV-2 infection, despite adequate immunization, should be taken into account to explain these differences.
In our study, we did not find differences in antibody titers of patients with more advanced liver disease, when compared to patients without previous decompensations, without portal hypertension or with CPT A, except for a nonsignificant trend to lower titers by 6 months. Although the small proportion of patients with CPT scores B and C compared with CPT A (21% vs. 79%) could have impacted these results, the effect of the stage of LC is controversial in the literature. Some studies have reported that patients with decompensated cirrhosis demonstrated suboptimal humoral (measured through anti-spike antibodies) and cellular immune responses against recombinant and inactivated COVID-19 vaccines [40] and that CPT B/C cirrhosis was an independent risk factor for negative neutralizing antibodies [41]. However, other studies reported no differences in antibody responses regarding compensated versus decompensated cirrhosis [30, 31] or according to MELD and CPT scores [32].
In our results, adenovirus vector vaccines were asso-ciated with lower antibody titers, compared to mRNA vaccines. These differences have been previously reported in the literature. In a meta-analysis on COVID-19 vaccine immunogenicity among CLD patients, including four studies on inactivated vaccines and three on mRNA vaccines, seroconversion rates were 86% in inactivated and 89% in mRNA vaccines [14]. In a study on CLD patients, the type of vaccine was associated with different humoral responses, which were lower for ChAdOx1-nCoV-19, followed by BNT162b2 mRNA and finally mRNA-1273, although these did not appear to associate with clinical efficacy [15]. Collier et al. [42] reported that mRNA-1273 vaccines provided initial high peak antibody responses that declined sharply by 6 months, whereas Ad26.COV2.S vaccines induced lower initial antibody responses which were relatively stable over time, and these differences in humoral kinetics could explain equivalent clinical responses. Similarly, in our study, these humoral differences were not related to lower clinical efficacy. A large case-control study in LC reported that differences between groups regarding vaccine clinical efficacy between Ad.26.COV2.S and mRNA vaccines were also not statistically significant [43].
An important limitation of this study was the fast vaccination rate and the prompt introduction of a booster dose in Portugal, which led to the exclusion of many patients who were already vaccinated at the time of recruitment and earlier termination of follow-up of the ones vaccinated with an additional dose. Also, we did not have a control group, and comparisons were made with available data from the general population from clinical trials and people included in real-life studies. Another limitation was the absence of an index antibody titer assessment before vaccination and, therefore, the impossibility to ascertain previously unknown asymptomatic SARS-CoV-2 infection before vaccination, which would affect humoral and clinical outcomes. Finally, our immunological assessment only included the humoral response but not cellular responses to the vaccine, which are also known to play a role in the development of immunity against the virus.
The main strengths of this study are (1) its prospective nature with close follow-up of all the patients since enrollment during a median period of 7 months (whereas most of the literature available refers to retrospective cohorts); (2) the assessment of both humoral and clinical outcomes in every patient throughout time. We used the same standardized techniques for antibody titer mea-surement within one laboratory group during different well-specified timepoints in every patient. Furthermore, all the patients were monitored frequently for clinical endpoints regarding safety and efficacy, providing an adequate and detailed evaluation of this susceptible population.
In conclusion, COVID-19 vaccines in patients with LC were safe, resulting in no serious adverse events. The humoral and clinical responses were good, even when compared to results reported for the general population in trials and healthy controls in real-life studies. We did not find an association between humoral and clinical responses, suggesting that further vaccination boosters should not be decided based on antibody titers. The only associations with lower humoral responses were older age and adenovirus vector vaccines. The severity of liver disease did not have an impact on humoral responses, even though we did not evaluate cellular immune responses. These results highlight the important role of COVID-19 vaccination in this susceptible group of patients, as recommended by international guidelines and national policies, in order to prevent SARS-CoV-2 infection, a precipitating factor for cirrhosis decompensation and death.