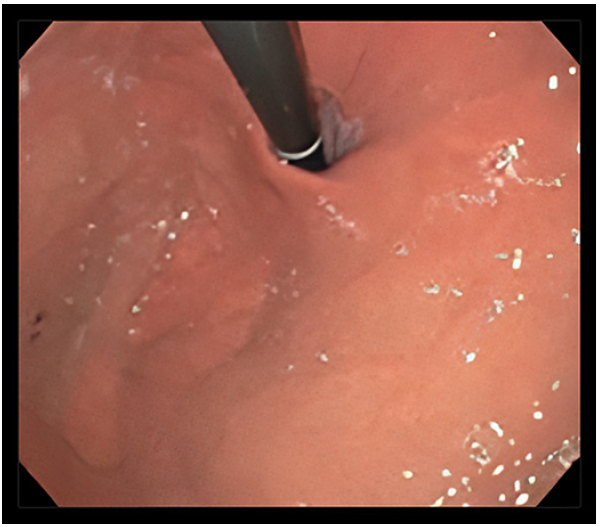
A 33-year-old man, with background of asthma, was referred to the gastroenterology outpatient clinic with daily heartburn and regurgitation over the last 2 years. No extra-esophageal reflux symptoms or dysphagia have been noted. Double-dose proton pump inhibitor (PPI) therapy for over 3 months was not successful. High-resolution esophageal manometry showed normal esophageal motility (Chicago Classification v4.0), normotensive lower esophageal sphincter (18.3 mm Hg), and normal integrated relaxation pressure (8.2 mm Hg). The 24-h pH-impedance test (off PPIs) revealed a pathological esophageal acid exposure time of 11.7% with a DeMeester score of 55.1. On impedance, the number of reflux episodes was 161, mainly acid refluxes, with positive symptomatic association (26 symptoms recorded by the patient). The Gastroesophageal Reflux Disease Health-Related Quality of Life Questionnaire (GERD-HRQL) score was 43 (ranges from 0-75) and the Frequency Scale for the Symptoms of GERD (FSSG) score was 35 (ranges from 0 to 48). Previous upper endoscopy showed grade B reflux esophagitis (Los Angeles classification) and grade II Hill’s flap valve (2-cm hiatus hernia, no lesions in the hernial sac) (Fig. 1). Esophageal biopsies have been performed, excluding eosinophilic esophagitis. After discussing with the patient about therapeutic options, he was unwilling to undergo surgery; therefore, anti-reflux mucosal ablation (ARMA) was proposed.
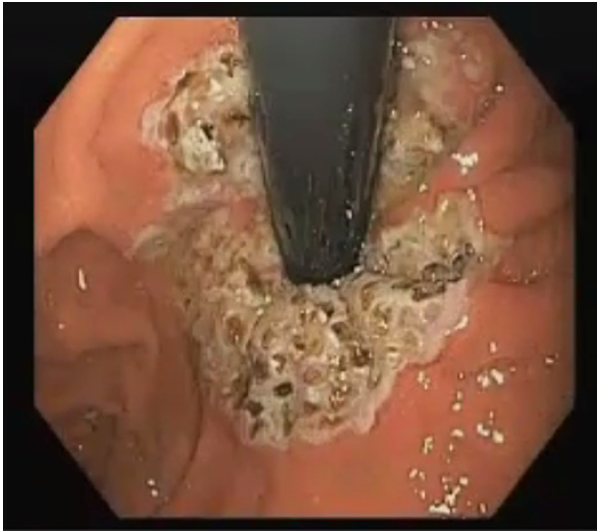
ARMA was performed using the triangle-tip knife (Olympus®) connected to an electrocautery generator (VIO300D; ERBE Elektromedizin, Tübingen, Germany) in spray coagulation mode (50 W, effect 2) (online suppl. video; for all online suppl. material, see https://doi.org/10. 1159/000535205). Mucosal ablation was performed around the cardia on the gastric side in a butterfly shape with a width of approximately 1.5 scope diameter, leaving two contralateral areas of normal cardia mucosa with approximately one scope diameter, to avoid stricture (Fig. 2). The procedure was done with propofol-based sedation, and no complications were noted. The patient received post-procedural double-dose PPIs for 2 months, with subsequent weaning of the medication.
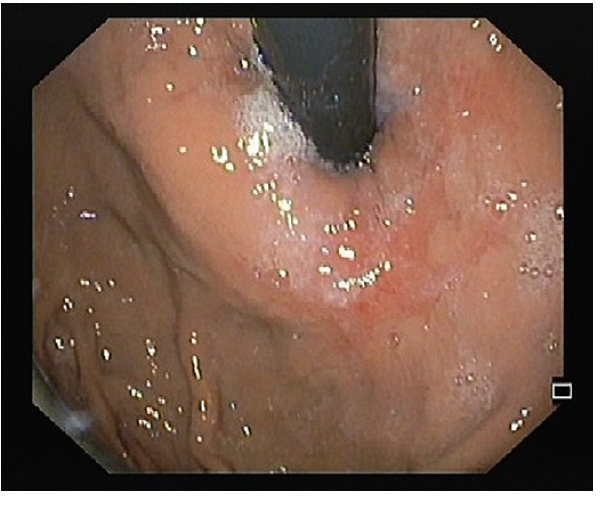
Three months later, he reported significant clinical improvement. The GERD-HRQL score was 21, and the FSSG score was 21. In addition, 24-h pH-impedance test showed normalization of esophageal parameters (acid exposure time: 0.9%, number of reflux episodes: 34, DeMeester score: 3.6). He underwent follow-up esophagogastroduodenoscopy, which showed resolution of the reflux esophagitis, with shrinking effect of the ablated area and grade I Hill’s flap valve (Fig. 3). Currently, 2 years post-procedure, the patient remains asymptomatic without PPIs (GERD-HRQL score of 7 and FSSG score of 5).
GERD is a highly prevalent condition that affects 8-33%of the worldwide population [1]. It can result in several serious complications, including esophageal stricture and Barrett’s esophagus, which may increase the risk of esophageal cancer. PPIs have been the cornerstone of the management of these patients. However, 30-40% of patients have persistent GERD symptoms despite PPI therapy [2]. The management of PPIs-refractory GERD has primarily included surgical laparoscopic fundoplication, after excluding other potential causes [3]. However, it is an invasive procedure with potential adverse events. Therefore, less invasive anti-reflux interventions are desired.
Transoral incisionless fundoplication (TIF) is a minimally invasive endoscopic option, which mirrors the Nissen fundoplication by using the EsophyX® device. TIF does not preclude future anti-reflux surgery and can be revised if required. However, it requires proprietary equipment and is a two-operator technique. Long-term outcomes of TIF 2.0 have been showing long-term elimination of GERD symptoms with no severe adverse events [4]. Nonablative radiofrequency treatment involves the application of radiofrequency energy to the muscle fibers of the lower esophageal sphincter and the gastric cardia through the Stretta® system. The procedure is usually safe and well tolerated, without compromising the possibility of future anti-reflux surgery. Nevertheless, outcome data are heterogeneous, displaying variable response rates [5]. Furthermore, it has been postulated that submucosal injection of inert substances into the gastroesophageal junction may cause tissue remodeling, resulting in improvement of GERD symptoms [6]. Several injectable agents have been evaluated; however, many are no longer available due to poor long-term efficacy and safety concerns. GERDx™ is a new endoscopic device that allows for full-thickness plication [7]. This two-operator technique has a relatively short operating time and a fast learning curve. The experience with the device is still minimal, and the very short follow-up makes this endoscopic treatment experimental at this time. In anti-reflux mucosectomy, endoscopic resection of the gastric cardiac mucosa is performed to reduce the opening of the gastroesophageal junction [8]. It does not require proprietary equipment. However, there is a considerable risk of perforation and bleeding. Given the lack of randomized controlled trials and long-term data, anti-reflux mucosectomy should be reserved for patients included in research protocols.
ARMA is a promising new endoscopic method for PPIs-refractory GERD, patients unwilling or unfitfor surgery, after excluding other potential causes of refractory reflux. Recent data have been demonstrating that it is well tolerated and results in gastroesophageal reflux suppression [9]. Moreover, it does not require costly add-on devices and can be performed in a standard endoscopy room. Another important strength of ARMA is that it can be repeated despite presence of fibrosis from previous therapies.
In this case, narrowing the cardia opening using ARMA was a safe and clinical successful procedure, with improved GERD-related symptoms and objective acid reflux parameters. In fact, this technique has been described in the literature as generally effective and safe in patients with refractory GERD, with a significant decrease in the esophageal acid exposure time, number of acid refluxes, and DeMeester score [10]. Nevertheless, randomized comparative studies with long-term follow-up are needed to address the efficacy of this technique.