Introduction
Prevalence of different gastroduodenal lesions detected at upper endoscopy is changing over time due to modifications of the main involved aggressive or protective factors. Indeed, prevalence of Helicobacter pylori infection - namely, the main aetiologic factor for peptic ulcer (gastric and duodenal) and gastric neoplastic lesions (low-grade B-cell MALT lymphoma, diffuse large B-cell lymphoma, adenocarcinoma) - is decreasing in developed countries [1-3]. Conversely, therapy with non-steroidal anti-inflammatory drugs (NSAIDs) - either as antiaggregant therapy or treatment of chronic rheumatic diseases and chronic pain - is largely used in routine practice, even as out-the-counter therapy [4-7], and its role in damaging gastric mucosa is well documented [8].
Similarly, prevalence of obesity is relentlessly increasing worldwide, and its association with gastro-oesophageal reflux disease (GORD) is recognized [9]. On the other hand, use of proton-pump inhibitors (PPI), the most effective drugs to treat lesions of acid-related diseases, has hugely increased in the last decades [10]. Since gastric cancer remains one of the most prevalent cancer-related causes of mortality and population-based screening programmes are not implemented in Western countries, to search for precancerous lesions in the stomach during endoscopy performed in routine practice is advised to identify patients deserving follow-up [11]. Current guidelines suggest to standard biopsies during appropriate upper endoscopies to assess the presence of extensive atrophy/metaplasia - i.e., involving both antral and gastric body mucosa - on gastric mucosa, because these precancerous lesions distinctly increase gastric cancer risk [11, 12]. Based on these observations, it is clinically worthy updating the diagnostic yield of upper endoscopies performed in routine practice. Indeed, previous large studies on prevalence of endoscopic and histological lesions in routine upper endoscopy performed in our country were published more than 10 years ago [13, 14].
The relevance of this topic is further strengthened when considering that more than 2.5 million of upper endoscopies was performed in Italy yearly, 1.2 million in UK, and 6.9 million in USA [15-17], and that the rate of inappropriate procedures was higher than 20% [18]. Therefore, we performed a multicentre study to assess both macroscopic and histological lesions currently de-tected at upper endoscopy performed in routine practice.
Materials and Methods
Study Design and Patients
In this cross-sectional study, clinical, endoscopic, and histological data of consecutive adult patients referred for upper endoscopy in the participating centres between October 1 and October 31, 2022, were anonymously reviewed and gathered in a specific Excel database. To better describe routine clinical practice, only data of patients referred by their general practitioners were collected, while those of in-patients were excluded. Moreover, we have taken into account solely patients who underwent the first endoscopic examination for any indication, while endoscopic surveillance procedures not considered. Clinical data collection was focused on the main indication for endoscopy, and ongoing therapies with antithrombotic drugs (antiaggregant or anticoagulant) and PPI. PPI therapy was defined as ongoing when drugs were taken until the previous day before or suspended less than 7 days before endoscopy. Alarm symptoms included anaemia, melena, persistent vomiting, weight loss, and dysphagia, as suggested in guidelines [19, 20]. Data on oesophageal, gastric, and duodenal lesions detected at endoscopy were computed, and a threshold age of 50 years was chosen for comparison, as suggested by Italian guidelines [12, 20]. Histological reports were reviewed to evaluate the prevalence of both H. pylori infection and that of extensive precancerous lesions (atrophic or metaplastic pangastritis) in the stomach.
Statistical Analysis
Frequencies, means, or medians were computed, with their 95%confidence intervals (CIs) and the odds ratios (ORs) calculated for the main observations. Comparison among subgroups was performed by using the χ2 test with Yate’s correction. A p value <0.05 was considered statistically significant.
Results
Descriptive Analysis
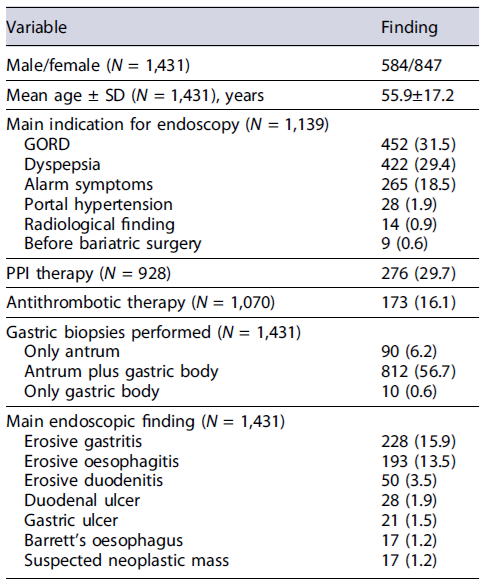
A total of 1,431 patients (M/F: 584/847; mean age: 55.9 ± 17.2) underwent first endoscopic examination in the 28 participating centres, including 24 community hospitals and 4 academic hospitals. The main indication for endoscopy was GORD (N = 452; 31.5%), dyspepsia (N = 422; 29.4%), alarm symptoms (N = 265; 18.5%), searching for gastro-oesophageal varices (N = 28; 1.9%), suspect radiological finding (N = 14; 0.9%), and evaluation before bariatric surgery (N = 9; 0.6%), while the information was lacking for the remaining 292 (20.4%) cases. There were 276 (29.7%) out of 928 patients in ongoing PPI therapy, and 173 (16.1%) out of 1,070 patients in antithrombotic, with either antiaggregant (N = 135) or anticoagulant (N = 38) therapy, when considering only cases with available information (Table 1).
Endoscopic Findings
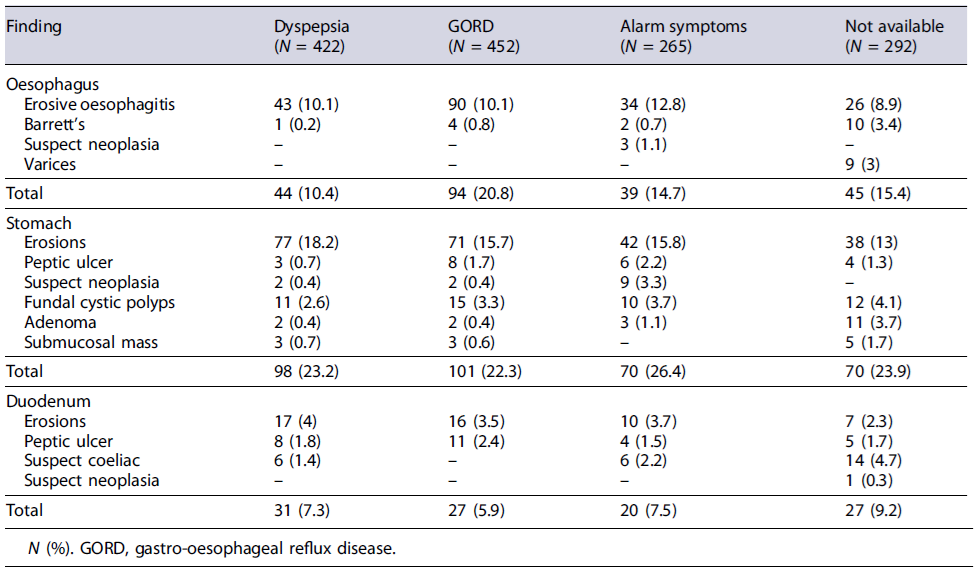
At endoscopic examination, at least one lesion in the oesophagus, stomach, and duodenum was detected in 222 (15.5%, 95% CI: 13.6-17.3), 339 (23.6%, 95% CI: 21.4-25.8), and 105 (7.3%, 95% CI: 5.9-8.6) patients, respectively. In detail, erosive oesophagitis or Barrett’s oesophagus was diagnosed in 193 (13.5%) and 17 (1.2%) cases, gastric erosions in 228 (15.9%), gastric ulcer in 21 (1.5%), duodenal erosions in 50 (3.5%), duodenal ulcer in 28 (1.9%), and coeliac disease in 26 (3.8%) cases, while a neoplastic lesion was overall suspected in 17 (1.2%) cases (Table 1). Therefore, the overall peptic ulcer prevalence was 3.4% (95% CI = 2.5-4.4). The endoscopic lesions according to the main indication for upper endoscopy are listed in Table 2.
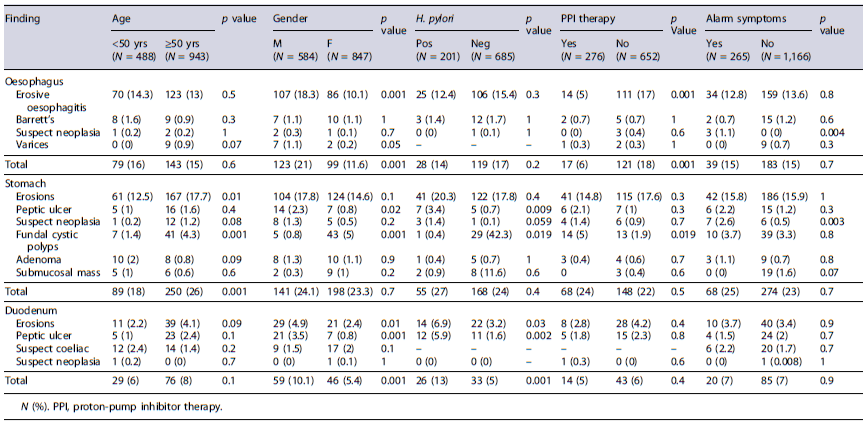
The distribution of different endoscopic findings according to patients’ age, gender, H. pylori infection, PPI therapy, and alarm symptoms is provided in Table 3. As shown, the overall endoscopic lesions in the stomach were more prevalent in patients aged ≥50 years (26% vs. 18%; p = 0.001; OR: 1.61; 95% CI: 1.23-2.12). Gastric ulcers (3.4% vs. 0.7%; p = 0.009), duodenal ulcers (5.9% vs. 1.6%; p = 0.002), and duodenal erosions (6.9%vs. 3.2%; p = 0.02) were more frequently detected in patients with H. pylori infection. Moreover, the prevalence of overall oesophageal (21% vs. 11.6%; p = 0.001; OR: 2.01; 95% CI: 1.51-2.69) and duodenal (10% vs. 5.4%; p = 0.001; OR: 1.95; 95% CI: 1.31-2.92) lesions was twice in males than in females, while fundal cystic polyps were predominant in females (5% vs. 0.8%; p = 0.001). Finally, the prevalence of overall neoplastic lesions was higher in those presenting with alarm symptoms (3.8% vs. 0.6%; p = 0.001; OR: 6.49; 95% CI: 2.4-17.2).
Histological Findings
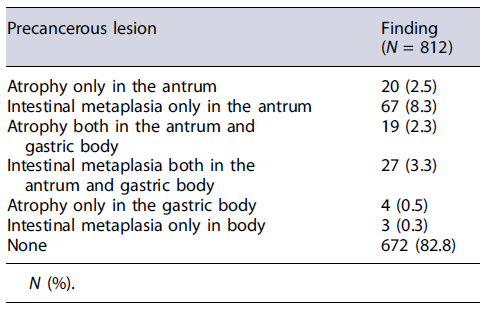
At histological assessment, H. pylori infection was overall detected in 201 (22.6%, 95% CI: 19.9-25.4) out of 886 in whom antral biopsies (with or without other sites) were taken, and in 185 (22.7%, 95% CI: 19.9-25.6) out of 812 patients for which standard (2 antral and 2 gastric bodies) biopsy sampling of the stomach was available. Prevalence of infection did not differ between patients aged <50 and ≥50 years (71/306, 23.2% vs. 130/580, 22.4%; p =0.8), nor between those taking PPI therapy or not (34/150, 22.6%vs. 99/388, 25.5%; p = 0.5). Taking into account data of only patients in whom a standard gastric biopsy sampling was available, the presence of extensive atrophy or metaplasia on gastric mucosa was overall detected in 46 (5.6%, 95% CI: 4-7.2) patients, including extensive atrophy in 19 (2.3%, 95% CI: 1.3-3.3) cases and extensive metaplasia in 27 (3.3%, 95% CI: 2-4.5) patients (Table 4). Prevalence of these extensive precancerous lesions was significantly higher (OR: 8.02; 95% CI: 2.46-26.11; p < 0.001) in patients aged ≥50 years (43/534, 8%) than in those <50 years (3/278, 1%), while it did not differ between patients with H. pylori infection and those without it (10/185, 5.4% vs. 35/616, 5.6%; p = 0.9). All the 3 (0.2%) suspected neoplastic lesions in the oesophagus were eventually diagnosed as adenocarcinoma, the 13 (0.9%) gastric masses were either carcinomas (N = 12) or MALT lymphoma (N = 1), and the single suspected duodenal lesion was an ampullary adenoma.
Discussion
This large, multicentre study updated data on prevalence of both endoscopic and main histological lesions detected at first upper endoscopy performed in routine practice. We observed that GORD symptoms were the most frequent indication for upper endoscopy, and that erosive oesophagitis was among the most frequent (13.5%) detected esions. Likewise, this mirrors the relentless increasing of GORD prevalence in the general population, at least in part, linked to the escalation of obesity incidence [21]. However, the prevalence of Barrett’s oesophagus (1.2%) we computed would appear not increased when compared to 2.2% and 1.6% reported on 2003 and 2012, respectively [14, 22]. We observed that prevalence of erosive oesophagitis was 3-fold lower in patients in ongoing PPI therapy and near halved in females, while it did not differ between young and older patients or those with or without H. pylori infection. Therefore, it may be speculated that an ongoing PPI therapy would reduce diagnosis of erosive oesophagitis leading undiagnosed several patients with a chronic or recurrent disease deserving an appropriate therapy and follow-up [10]. Indeed, current guidelines advise to interrupt PPI therapy at least 2 weeks before diagnostic upper endoscopy [19, 23], a procedure not followed in as many as 20% in our study, even if this value seems to be lower than 36.3-50.7%previously reported in other series [14, 24].
Regarding neoplastic lesions, our data found that the overall prevalence was distinctly higher in patients presenting with alarm symptoms than in those without, confirming that upper endoscopy should be promptly scheduled in patients presenting with these symptoms [19, 20]. In detail, we found that the frequency of oesophageal cancer remains 4-fold lower than that of neoplastic lesions in the stomach.
Another finding of our study was that the overall prevalence (3.4%) of peptic ulcer remained substantially stable when compared to 2.7-5.5% reported in other Italian series through 2010 and 2021 [14, 25]. This occurs even if the 22.6% prevalence of H. pylori observed in this study would appear distinctly lower than 34% previously reported in 2012 [14], suggesting a trend towards a relentless reduction of this infection in endoscopic series. Therefore, the expected decrease of peptic ulcer incidence due to the lower prevalence of H. pylori is probably offset by the more diffuse use of NSAIDs, even as out-the-counter use [4-7]. Indeed, H. pylori infection and NSAID use remain the main pathogenetic factors for peptic ulcer, idiopathic ulcer prevalence being negligible in Italy [13].
Standard sampling on gastric mucosa is advised during routine endoscopy to search for presence and extension of precancerous lesions in the stomach - namely, extensive atrophic or metaplastic gastritis [12, 26]. Our data found a 5.6% prevalence of these extensive precancerous lesions on gastric mucosa, a value consistent with data of previous Italian studies showing a prevalence ranging from 2.3% to 7.8% [27-30]. Therefore, by performing standard sampling of gastric mucosa during endoscopy, it is possible to identify the small subgroup of patients with extensive precancerous lesions in the stomach at increased risk of developing gastric cancer deserving a scheduled follow-up [11]. Regrettably, our data showed that standard biopsy sampling is performed in only half patients, suggesting that an implementation in routine practice is still needed.
Conclusions
Data of present study found that the overall endoscopic lesions were more prevalent in patients aged ≥50 years, peptic ulcer and erosions were more frequent in H. pylori-infected patients, and extensive gastric precancerous lesions deserving follow-up were present in less than 6% of cases.